Catalyzing Innovation with
Aquigen’s Impurity Standards
Categories
- Acetophenon (9)
- Acetovanillone (4)
- Alfuzosin (22)
- Algestone (7)
- API & Intermediates (118)
- Acebutolol (16)
- Acetylcysteine (26)
- Almotriptan (1)
- Apixaban (1)
- Colesevelam (1)
- Dabigatran (2)
- Deucravacitinib (1)
- Diacerein (1)
- Miscellaneous (1)
- Apigenin (1)
- Aprocitentan (1)
- Flufentacet (2)
- Frovatriptan (2)
- Impurity Standard (86)
- Impurity Standards (35325)
- 'Lenacapavir' related Reference Standards & Products (63)
- 'Nitroso' related Reference Standards & Products (1141)
- Abacavir (35)
- Abaloparatide (1)
- Abamectin (2)
- Abametapir (1)
- Abemaciclib (15)
- Abietic Acid (4)
- Abiraterone (90)
- Abrocitinib (4)
- Acalabrutinib (39)
- Acamprosate (5)
- Acarbose (10)
- Acebrophylline (2)
- Acediasulfone (1)
- Acedoben (2)
- Acemetacin (7)
- Acenocoumarol (1)
- Acesulfame Potassium (4)
- Acetazolamide (16)
- Acetylcholine (4)
- Acetylisovaleryltylosin (1)
- Acetyltributyl Citrate (4)
- Aciclovir (12)
- Acitretin (8)
- Aclonifen (5)
- Acoramidis (4)
- Acrivastine (9)
- Adagrasib (1)
- Adapalene (18)
- Adefovir (3)
- Ademethionine (1)
- Adenosine (21)
- Adiphenine (3)
- Adrenaline (14)
- Adrenalone (3)
- Afatinib (49)
- Aflatoxin (4)
- Afobazole (2)
- Agnuside (1)
- Agomelatin (29)
- Alarelin (1)
- Albendazole (1)
- Alcaftadine (13)
- Alclometasone Dipropionate (6)
- Aldicarb (1)
- Alectinib (7)
- Alendronate (9)
- Alfacalcidol (4)
- Alfadex (3)
- Alfaxalone (2)
- Alfentanil (9)
- Alimemazine Hemitartrate (6)
- Alitretinoin (2)
- Allantoin (3)
- Allopurinol (17)
- Allylestrenol (3)
- Almotriptan (26)
- Alogliptin (57)
- Alosetron (3)
- Alpelisib (1)
- Alprazolam (11)
- Alprostadil (12)
- Altiratinib (1)
- Altizide (5)
- Altrenogest (1)
- Altretamine (3)
- Alverine (17)
- Alvespimycin (2)
- Alvimopan (38)
- Amantadine (4)
- Ambrisentan (18)
- Ambroxol (21)
- Amfenac (1)
- Amfepramone (2)
- Amidotrizoic Acid (9)
- Amifampridine (4)
- Amikacin (11)
- Amiloride (6)
- Aminocaproic Acid (15)
- Aminolevulinic Acid Hydrochloride (4)
- Aminophylline (1)
- Amiodarone (26)
- Amitraz (4)
- Amitriptyline (16)
- Amlodipine (8)
- Amodiaquine (16)
- Amorolfine (15)
- Amoxapine (14)
- Amoxicillin (48)
- Ampelopsin (1)
- Amphetamine Sulfate (16)
- Amphotericin B (10)
- Ampicillin (34)
- Amprolium (2)
- Ampyrone (2)
- Amrubicin (2)
- Amsacrine (5)
- Amtolmetin Guacil (4)
- Amylmetacresol (11)
- Anagrelide (26)
- Anastrozole (36)
- Andrographolide (1)
- Anhydrotetracycline (1)
- Anidulafungin (8)
- Aniracetam (2)
- Annamycin (1)
- Antazoline (2)
- Anthracene (4)
- Anthraquinone (6)
- Antipyrine (7)
- Antroquinonol (1)
- Apalutamide (31)
- API & Intermediates (3)
- Apigenin (2)
- Apixaban (107)
- Apoatropine (1)
- Apomorphine (5)
- Apramycin (3)
- Apremilast (51)
- Aprepitant (39)
- Aprindine (4)
- Aprobarbital (1)
- Aprocitentan (2)
- Arbutin (1)
- Argatroban (25)
- Arginine (5)
- Aripiprazole (74)
- Armodafinil (3)
- Arotinolol (2)
- Arteether (2)
- Artemether (15)
- Artemisinin (23)
- Arterolane (2)
- Artesunate (10)
- Asciminib (62)
- Ascorbic acid (14)
- Asenapine (28)
- Aspartame (9)
- Aspartic Acid (10)
- Aspirin (20)
- Astemizole (5)
- Ataluren (9)
- Atenolol (22)
- Atezolizumab (1)
- Atipamezole (5)
- Atogepant (22)
- Atomoxetine (23)
- Atomoxetine Nitroso (6)
- Atorvastatin (124)
- Atosiban (7)
- Atovaquone (17)
- Atracurium (30)
- Atrazine (1)
- Atripla (1)
- Atropine (18)
- Auristatin (3)
- Avacopan (2)
- Avanafil (39)
- Avapritinib (4)
- Avatrombopag (7)
- Avermectin (9)
- Avibactam (8)
- Avilamycin (3)
- Avobenzone (1)
- Axitinib (44)
- Azacitidine (49)
- Azadirachtin (1)
- Azafenidin (1)
- Azathioprine (11)
- Azelaic acid (10)
- Azelastine (28)
- Azelnidipine (1)
- Azilsartan (77)
- Azilsartan Medoxomil (1)
- Azithromycin (35)
- Azoxystrobin (1)
- Aztreonam (18)
- Bacampicilli (10)
- Bacitracin (23)
- Baclofen (40)
- Bacoside (5)
- Baicalin (2)
- Baloxavir Marboxil (31)
- Balsalazide (13)
- Bambuterol (10)
- Bamifylline (1)
- Barnidipine (8)
- Bazedoxifene (13)
- Beclomethasone (41)
- Bedaquiline (22)
- Belinostat (5)
- Belumosudil (8)
- Belzutifan (13)
- Bemcentinib (1)
- Bemotrizinol (1)
- Bempedoic Acid (52)
- Benazepril Hydrochloride (19)
- Bendamustine (60)
- Bendroflumethazide (4)
- Benethamine Penicillin (2)
- Benfotiamine (5)
- Benidipine (13)
- Benperidol (6)
- Benserazide (9)
- Benzalkonium Chloride (6)
- Benzatropine (5)
- Benzbromarone (26)
- Benzenesulfonic Acid (6)
- Benzethonium Chloride (1)
- Benzetimide Hydrochloride (1)
- Benzgalantamine (2)
- Benzhydrylamine (1)
- Benzocaine (40)
- Benzoic Acid (20)
- Benzonatate (7)
- Benzoyl Peroxide (9)
- Benzphetamine (2)
- Benzydamine (15)
- Benzyl Benzoate (1)
- Bepotastine (16)
- Beraprost (9)
- Berberine (1)
- Berotralstat (6)
- Besifloxacin (2)
- Betacarotene (1)
- Betahistine (24)
- Betaine (1)
- Betamethasone (102)
- Betamethasone Sodium Phosphate (20)
- Betaxolol (8)
- Bethanechol (2)
- Betrixaban (14)
- Bevirimat (1)
- Bexagliflozin (25)
- Bexarotene (14)
- Bezafibrate (9)
- Biapenem (5)
- Bibrocathol (1)
- Bicalutamide (33)
- Bictegravir (23)
- Bifenthrin (2)
- Bifonazole (6)
- Bilastine (54)
- Bimatoprost (27)
- Binimetinib (32)
- Biotin (20)
- Biperiden Hydrochloride (11)
- Bisabolol (4)
- Bisacodyl (8)
- Bismuth Subsalicylate (1)
- Bisoctrizole (2)
- Bisoprolol (62)
- Bivalirudin (4)
- Bleomycin Sulfate (19)
- Blonanserin (4)
- Boldenone Undecylenate (1)
- Boldine (7)
- Bortezomib (90)
- Boscalid (3)
- Bosentan (29)
- Bosutinib (27)
- Boswellic acid (5)
- Brassicasterol (1)
- Brassinolide (5)
- Bremelanotide (1)
- Brexpiprazole (2)
- Brigatinib (13)
- Brimonidine (54)
- Brinzolamide (30)
- Brivanib (4)
- Brivaracetam (70)
- Brivudine (11)
- Brodifacoum (1)
- Bromadiolone (1)
- Bromazepam (8)
- Brombuterol Hydrochloride (1)
- Bromfenac (33)
- Bromhexine (11)
- Bromocriptine (3)
- Bromocriptine mesilate (9)
- Bromopride (7)
- Brompheniramine (9)
- Bronopol (3)
- Brotizolam (2)
- Bucetin (1)
- Bucindolol (1)
- Buclizine (4)
- Budesonide (52)
- Bufotenine (3)
- Bufuralol (1)
- Bumetanide (16)
- Bupivacaine (32)
- Buprenorphine (21)
- Bupropion (66)
- Busalfan (9)
- Buserelin (1)
- Buspirone (26)
- Butalbital (1)
- Butamirate (15)
- Butenafine (5)
- Butoconazole (10)
- Butorphanol (2)
- Butropium bromide (1)
- Butylhydroxyanisole (3)
- Butylhydroxytoluene (6)
- Buy Empagliflozin Impurities (113)
- Cabazitaxel (57)
- Cabergoline (14)
- Cabotegravir (15)
- Cabozantinib (38)
- Caffeic Acid (4)
- Caffeine (20)
- Calanolide (1)
- Calcifediol (9)
- Calcipotriol (13)
- Calcitonin Salmon (43)
- Calcitriol (8)
- Calcobutrol Sodium (1)
- Campesterol (1)
- Camphor (2)
- Camptothecin (23)
- Camylofin (3)
- Canagliflozin (67)
- Candesartan (55)
- Candicidin (1)
- Cangrelor (6)
- Cannabigerol (2)
- Capecitabine (33)
- Capmatinib (3)
- Capsaicin (11)
- Captopril (27)
- Carbamazepine (30)
- Carbazochrome (4)
- Carbetocin (6)
- Carbidopa (23)
- Carbimazole (3)
- Carbinoxamine (6)
- Carbocisteine (7)
- Carboplatin (25)
- Carboprost Tromethamine (5)
- Carbovir (1)
- Carebastine (3)
- Carfilzomib (98)
- Carglumic Acid (18)
- Cariprazine (38)
- Carisoprodol (24)
- Carmustine (3)
- Carprofen (14)
- Carvedilol (46)
- Carvone (2)
- Caryophyllene oxide (2)
- Cascaroside (6)
- Caspofungin (28)
- Catharanthine (1)
- Cathine (1)
- Cedazuridine (1)
- Cefacetrile (6)
- Cefaclor (22)
- Cefadroxil (21)
- Cefalexin (18)
- Cefalonium (2)
- Cefalotin (9)
- Cefazedone (4)
- Cefazolin (26)
- Cefdinir (20)
- Cefditoren Pivoxil (2)
- Cefepime (14)
- Cefetamet (4)
- Cefiderocol (2)
- Cefixime (26)
- Cefoperazone (20)
- Cefotaxime Sodium (11)
- Cefotiam (2)
- Cefoxitin (17)
- Cefozopran (1)
- Cefpirome (2)
- Cefpodoxime Proxetil (16)
- Cefprozil Monohydrate (25)
- Cefradine (6)
- Ceftaroline (17)
- Ceftazidime (18)
- Ceftibuten (2)
- Ceftiofur (13)
- Ceftobiprole (1)
- Ceftolozane (2)
- Ceftriaxone (19)
- Cefuroxime (39)
- Celecoxib (37)
- Celiprolol Hydrochloride (14)
- Cephapirin (5)
- Ceramide (4)
- Ceritinib (17)
- Cerivastatin Sodium (2)
- Cetilistat (1)
- Cetirizine (52)
- Cetrimide (1)
- Cetrorelix (19)
- Cetylpyridinium (4)
- Cevimeline (12)
- Chenodeoxycholic Acid (17)
- Chicoric Acid (1)
- Chlorambucil (37)
- Chloramphenicol (5)
- Chlordiazepoxide (7)
- Chlorhexidine (19)
- Chlorhexidine Gluconate Solution (1)
- Chlormadinone (20)
- Chlorobutanol (7)
- Chlorocresol (3)
- Chloroquine phosphate (5)
- Chloroxylenol (3)
- Chlorphenamine (23)
- Chlorphenesin (1)
- Chlorpromazine (27)
- Chlorpropamide (2)
- Chlorprothixene (7)
- Chlorpyrifos (1)
- Chlorquinaldol (2)
- Chlortalidone (30)
- Chlorthion (1)
- Chlorzoxazone (3)
- Cholecalciferol (20)
- Cholesterol (36)
- Choline Chloride (1)
- Cibenzoline (2)
- Ciclesonide (8)
- Ciclopirox (5)
- Cidofovir (9)
- Cilastatin (20)
- Cilazapril (7)
- Cilnidipine (15)
- Cilostazol (24)
- Cimetidine (20)
- Cinacalcet (74)
- Cinchocaine (6)
- Cinchonidine (3)
- Cinitapride (2)
- Cinnarizine (9)
- Ciprofibrate (6)
- Ciprofloxacin (35)
- Cisapride (6)
- Cisatracurium (47)
- Cisplatin (10)
- Citalopram (44)
- Citicoline (8)
- Cladribine (11)
- Clarithromycin (25)
- Clascoterone (3)
- Clavulanate (22)
- Clemastine (8)
- Clemizole (4)
- Clenbuterol (8)
- Clevidipine (11)
- Clidinium Bromide (8)
- Clindamycin (1)
- Clindamycin Phosphate (87)
- Clioquinol (4)
- Clobenzorex (2)
- Clobetasol (25)
- Clobetasone Butyrate (9)
- Clobutinol Hydrochloride (6)
- Clodronate (4)
- Clofarabine (24)
- Clofazimine (12)
- Clomiphene (36)
- Clomipramine (54)
- Clonazepam (16)
- Clonidine (20)
- Clonixin (11)
- Cloperastine (3)
- Clopidogrel (58)
- Cloprostenol (3)
- Clorazepate (4)
- Clorsulon (1)
- Closantel (11)
- Clothianidin (3)
- Clotrimazole (13)
- Cloxacillin (11)
- Cloxazolam (3)
- Clozapine (24)
- Cobicistat (34)
- Cobimetinib (5)
- Cocaine (4)
- Codeinone (1)
- Colchicine (22)
- Colesevelam (22)
- Colestipol (6)
- Colfosceril Palmitate (4)
- Colistimethate (1)
- Copanlisib (7)
- Cornigerine (1)
- Cortexolone (6)
- Corticosterone (2)
- Cortisone (2)
- Corydaline (1)
- Cosyntropin (1)
- Coumarin (2)
- Coumatetralyl (1)
- Creatine (4)
- Creatinine (1)
- Crisaborole (20)
- Crizotinib (7)
- Cromolyn (4)
- Crotamiton (2)
- Curcumin (5)
- Cyamemazine (3)
- Cyantraniliprole (6)
- Cyclamate (7)
- Cyclizine (5)
- Cyclobenzaprine (13)
- Cyclopentolate (5)
- Cyclophosphamide (47)
- Cycloserine (8)
- Cyclosporin (21)
- Cyhalothrin (1)
- Cynocobalamin (20)
- Cyprodinil (2)
- Cyproheptadine (15)
- Cyproterone Acetate (17)
- Cysteamine (8)
- Cytarabine (15)
- Cytidine (5)
- Cytisine (3)
- Cytosine (9)
- Dabigatran (120)
- Dabrafenib (3)
- Dacarbazine (8)
- Daclatasvir (38)
- Dacomitinib (11)
- Dactinomycin (1)
- Daidzin (1)
- Dalargin (1)
- Dalbavancin (15)
- Dalfampridine (11)
- Danicopan (11)
- Danofloxacin (1)
- Dantrolene (12)
- Dapagliflozin (120)
- Dapivirine (1)
- Dapoxetine (20)
- Dapsone (35)
- Daptomycin (50)
- Daridorexant (3)
- Darifenacin (60)
- Darolutamide (13)
- Darunavir (87)
- Dasatinib (62)
- Daunorubicin (13)
- Decitabine (73)
- Deferasirox (38)
- Deferiprone (8)
- Deferoxamine (16)
- Deflazacort (13)
- Degarelix (2)
- Delafloxacin (11)
- Delamanid (7)
- Delorazepam (2)
- Deltamethrin (2)
- Demeclocycline (10)
- Denaverine (1)
- Deoxycholic Acid (2)
- Deoxynivalenol (1)
- Dequalinium Chloride (4)
- Deracoxib (6)
- Desipramine (14)
- Desloratadine (34)
- Desmopressin (20)
- Desmosterol Sulfate (2)
- Desogestrel (16)
- Desonide (12)
- Desoximetasone (7)
- Desoxycorticosterone Acetate (1)
- Desvenlafaxine (22)
- Detomidine (6)
- Deucravacitinib (11)
- Dexamethasone (69)
- Dexamethasone Sodium Phosphate (3)
- Dexamfetamine Sulfate (7)
- Dexbrompheniramine Maleate (1)
- Dexlansoprazole (17)
- Dexmethylphenidate (1)
- Dexpanthenol (7)
- Dexrazoxane (15)
- Dextromethorphan (23)
- Dextropropoxyphene (7)
- Diacerein (11)
- Diafenthiuron (1)
- Diatrizoic Acid (3)
- Diaveridine (1)
- Diazepam (1)
- Diazinon (1)
- Diazoxide (6)
- Dichlorvos (1)
- Diclazuril (6)
- Diclofenac (56)
- Diclofenamide (4)
- Dicloxacillin (5)
- Dicyclomine (1)
- Dicycloverine (14)
- Didanosine (10)
- Dienogest (27)
- Diethylcarbamazine (3)
- Diethylstilbestrol (1)
- Diethyltoluamide (2)
- Difamilast (1)
- Difelikefalin (10)
- Difenacoum (4)
- Difenoconazole (1)
- Diflorasone (5)
- Difloxacin Hydrochloride Trihydrate (9)
- Diflubenzuron (2)
- Diflucortolone Valerate (15)
- Diflunisal (13)
- Difluprednate (29)
- Digoxin (13)
- Dihydralazine (5)
- Dihydroartemisinin (10)
- Dihydrocodeine (6)
- Dihydroergocristine Mesylate (15)
- Dihydroergotamine (10)
- Dihydrostreptomycin sulfate (7)
- Dihydrotachysterol (4)
- Diltiazem (37)
- Dimenhydrinate (17)
- Dimethoate (2)
- Dimetindene (16)
- Dinoprost (6)
- Dinoprostone (7)
- Dinotefuran (3)
- Diosgenin (2)
- Diosmetin (5)
- Diosmin (15)
- Dioxybenzone (1)
- Diphenhydramine (18)
- Diphenidol (2)
- Diphenoxylate (10)
- Dipivefrin (5)
- Diprophylline (5)
- Dipyridamole (17)
- Diquafosol (4)
- Dirithromycin (2)
- Diroximel Fumarate (13)
- Disopyramide (1)
- Disulfiram (6)
- Disuprazole (1)
- Dithranol (5)
- Dityrosine (2)
- Diuron (2)
- Dobesilic Acid (1)
- Dobutamine (19)
- Docetaxel (59)
- Docosahexaenoic Acid (6)
- Docusate Sodium (8)
- Dofetilide (23)
- Dolasetron (7)
- Dolutegravir (49)
- Domiphen (1)
- Domperidone (25)
- Donepezil (57)
- Dopamine (18)
- Doravirine (7)
- Doripenem (1)
- Dorzolamide (22)
- Dosulepin (30)
- Dotinurad (1)
- Dovramilast (1)
- Doxazosin (21)
- Doxepin (23)
- Doxercalciferol (7)
- Doxofylline (5)
- Doxorubicin (29)
- Doxorubicinol (3)
- Doxycycline (16)
- Doxylamine (16)
- Dronedarone (26)
- Droperidol (13)
- Dropropizine (9)
- Drospirenone (28)
- DROTAVERINE (8)
- Droxidopa (34)
- Duloxetine (54)
- Dutasteride (20)
- Duvelisib (2)
- Dyclonine (2)
- Dydrogesterone (12)
- Ebastine (15)
- Eberconazole (7)
- Ecamsule (8)
- Ecgonine (2)
- Echimidine (2)
- Econazole (6)
- Edaravone (39)
- Edoxaban (166)
- Efavirenz (29)
- Efinaconazole (57)
- Efonidipine (2)
- Eicosapentaenoic Acid (37)
- Elacestrant (13)
- Elafibranor (10)
- Elagolix Sodium (62)
- Elaiophylin (1)
- Eldecalcitol (6)
- Eletriptan (37)
- Elexacaftor (5)
- Eliglustat (28)
- Elmustine (6)
- Elobixibat (24)
- Eltrombopag (64)
- Eltrombopag Olamine (2)
- Eluxadoline (25)
- Elvitegravir (9)
- Emedastine (7)
- Emetine (2)
- Emrusolmin (1)
- Emtricitabine (67)
- Enalapril (32)
- Enalaprilat Dihydrate (11)
- Enasidenib (2)
- Encorafenib (12)
- Enoxolone (6)
- Enramycin (2)
- Enrofloxacin (11)
- Ensifentrine (4)
- Ensulizole (1)
- Entacapone (24)
- Entecavir (35)
- Enzalutamide (72)
- Epalrestat (10)
- Eperisone (3)
- Ephedrine Hydrochloride (14)
- Epicatechin (3)
- Epigallocatechin Gallate (1)
- Epiisopiloturine (1)
- Epinastine (14)
- Epinephrine (41)
- Epirubicin (12)
- Epitalon (1)
- Eplerenone (16)
- Epoprostenol (11)
- Epoxiconazole (1)
- Eprosartan (17)
- Eptifibatide (5)
- Equilenin (5)
- Equilin (15)
- Eravacycline (5)
- Erdafitinib (5)
- Erdosteine (16)
- Ergocalciferol (13)
- Ergotamine (3)
- Eribulin (20)
- Eriocitrin (2)
- Erlotinib (75)
- Ertapenem (33)
- Ertugliflozin (23)
- Erucifoline (2)
- Erythromycin (48)
- Esaxenone (3)
- Escin (3)
- Escitalopram (50)
- Esketamine (6)
- Eslicarbazepine Acetate (8)
- Esmolol (17)
- Esomeprazole (40)
- Estetrol (26)
- Estradiol (51)
- Estradiol Valerate (15)
- Estrone (26)
- Etamsylate (2)
- Etelcacetide (1)
- Ethacrynic Acid (15)
- Ethambutol (9)
- Ethinyl Estradiol (26)
- Ethionamide (7)
- Ethopabate (5)
- Ethopropazine (1)
- Ethosuximide (6)
- Ethotoin (1)
- Ethylestrenol (1)
- Ethylmorphine (1)
- Etifoxine (15)
- Etodolac (45)
- Etofenamate (13)
- Etomidate (13)
- Etonogestrel (28)
- Etoposide (26)
- Etoricoxib (42)
- Etrasimod (19)
- Etravirine (33)
- Etylhexyl Triazone (4)
- Etynodiol (1)
- Eugenol (2)
- Eupatilin (1)
- Europine (2)
- Everolimus (21)
- Evocalcet (15)
- Exametazime (2)
- Exatecan (2)
- Exemestane (25)
- Ezetimibe (121)
- Famciclovir (30)
- Famotidine (49)
- Faropenem (8)
- Favipiravir (22)
- Febantel (5)
- Febuxostat (84)
- Fedratinib (4)
- Felbamate (7)
- Felodipine (9)
- Fenazaquin (1)
- Fenbendazole (6)
- Fenchone (7)
- fenerenone (1)
- Fenfluramine (9)
- Fenitrotion (1)
- Fenofibrate (37)
- Fenoldopam (4)
- Fenoprofen (11)
- Fenoterol (7)
- Fenoverine (1)
- Fenoxaprop (4)
- Fenpipramide (1)
- Fenpiverinium (1)
- Fenpyroximate (2)
- Fenspiride (5)
- Fentanyl (27)
- Fenticonazole (8)
- Ferric maltol (2)
- Ferulic Acid (2)
- Fesoterodine (48)
- Fexofenadine (57)
- Fexuprazan (3)
- Fezolinetant (15)
- Fibanserin (5)
- Fidaxomicin (16)
- Fimasartan (7)
- Finasteride (18)
- Finerenone (32)
- Fingolimod (70)
- Fipronil (3)
- Firocoxib (10)
- Flavomycin (1)
- Flavoxate (6)
- Flecainide (2)
- Flomoxef (1)
- Flonicamid (1)
- Florasulam (2)
- Florbetaben-(18F) (2)
- Florbetapir (2)
- Florfenicol (8)
- Flortaucipir (F-18) (1)
- Fluazinam (2)
- Fluazuron (1)
- Flubendazole (8)
- Flucloxacillin (19)
- Fluconazole (37)
- Flucortolone (2)
- Fludarabine Phosphate (15)
- Fludeoxyglucose (3)
- Fludrocortisone Acetate (1)
- Flumazenil (10)
- Flumequine (3)
- Flumethasone (9)
- Flumethrin (1)
- Flumioxazin (1)
- Flunarizine (6)
- Flunisolide (6)
- Flunitrazepam (6)
- Fluocinolone (23)
- Fluocinonide (8)
- Fluorescein (9)
- Fluoxetine (35)
- Fluoxymesterone (1)
- Flupentixol (11)
- Fluphenazine (1)
- Fluphenazine Decanote (9)
- Fluprbiprofen (17)
- Fluprednidene Acetate (9)
- Fluralaner (5)
- Flurandrenolide (3)
- Flurazepam (8)
- Flurometholone (25)
- Fluroxypyr (1)
- Flutamide (9)
- Flutemetamol-(18F) (2)
- Fluticasone (81)
- Flutrimazole (4)
- Fluvoxamine (41)
- Folic Acid (39)
- Folinate Hydrate (28)
- Fondaparinux (6)
- Formoterol (3)
- Fosamprenavir (16)
- Fosaprepitant (1)
- Foscarnet (5)
- Fosdenopterin (19)
- Fosfomycin (20)
- Fosinopril (17)
- Fosnetupitant (12)
- Fosphenytoin Sodium (6)
- Fosravuconazole (5)
- Fostemsavir (1)
- Fructose (1)
- Frullanolide (2)
- Fruquintinib (9)
- Furagin (1)
- furazidin (1)
- Furazolidone (1)
- Furosemide (14)
- Fusidic Acid (18)
- Futibatinib (11)
- Gabalactam (3)
- Gabapentin (71)
- Gadobutrol (5)
- Gadodiamide (5)
- GADOPENTETATE (1)
- Gadoterate (2)
- Galantamine (15)
- GALAXOLIDE (2)
- Gamithromycin (12)
- Ganaxolone (3)
- Ganciclovir (29)
- GANIRELIX (11)
- Gatifloxacin (26)
- Gefapixant (5)
- Gefitinib (47)
- Gemcitabine (34)
- GEMEPROST (1)
- Gemfibrozil (13)
- Gemigliptin (6)
- GENISTIN (2)
- Gentamicin (25)
- Gestodene (18)
- GILTERITINIB (10)
- Gimeracil (7)
- Gingerol (1)
- Ginkgolide (4)
- GINSENOSIDE (3)
- Givinostat (4)
- Glabridin (1)
- Glasdegib (2)
- GLATIRAMER ACETATE (1)
- Glecaprevir (4)
- Glibenclamide (11)
- Gliclazide (17)
- Glimepiride (36)
- Glipizide (30)
- Gluconic Acid (3)
- Glutamic Acid (13)
- Glutamine (4)
- Glutathione (6)
- Glycine (11)
- Glycocholic Acid (6)
- Glycodeoxycholic Acid (1)
- Glycopyrrolate (19)
- Glycopyrronium Bromide (19)
- Glycyrrhizinate (2)
- Glyphosate (1)
- Gonadorelin (1)
- Goserelin (16)
- Granisetron (16)
- Grapiprant (1)
- Grazoprevir Impurity (1)
- Griseofulvin (2)
- Guaifenesin (14)
- Guanabenz (1)
- Guanadrel (1)
- Guanfacine (22)
- Guanine (2)
- Halcinonide (21)
- Halobetasol (20)
- Halofuginone Lactate (1)
- Haloperidol (38)
- Harmine (1)
- Harpagoside (1)
- Hederacoside C (1)
- Heliotrine (2)
- Heparin (10)
- Hesperetin (12)
- Hesperidin (2)
- Hexetidine (10)
- Hexoprenaline (2)
- Hidiosmin (1)
- Histamine (3)
- Histidine (7)
- Homatropine Hydrobromide (9)
- Homopterocarpin (1)
- Homosalate (1)
- Hycosamide (1)
- Hydralazine (23)
- Hydrochlorothiazide (45)
- Hydrocodone (12)
- Hydrocortisone (115)
- Hydroflumethiazide (8)
- Hydromorphone (7)
- Hydroquinone (4)
- Hydroxocobalamin Acetate (7)
- Hydroxychloroquine (81)
- Hydroxyethyl Salicylate (1)
- Hydroxyzine (22)
- Hymecromone (4)
- Hyoscine Butylbromide (14)
- Hyoscine Hydrobromide (6)
- Hyoscyamine (4)
- Hypericin (1)
- Ibandronate (17)
- Ibogaine (1)
- Ibrutinib (88)
- Ibuprofen (93)
- Ibutilide (4)
- Icatibant (17)
- Icotinib (1)
- Idarubicin (10)
- Idebenone (6)
- Idelalisib (9)
- Ifosfamide (14)
- Iguratimod (4)
- Ilaprazole (20)
- Iloperidone (31)
- Iloprost (3)
- Imatinib (61)
- Imeglimin (7)
- Imidacloprid (8)
- Imidapril (3)
- Imidocarb (2)
- Iminostilbene (5)
- Imipenem (12)
- Imipramine (5)
- Imiquimod (15)
- Inavolisib (4)
- Indacaterol (49)
- Indapamide (30)
- Indigo Carmine (4)
- Indinavir (1)
- Indocyanine Green (5)
- Indomethacin (30)
- Indoramin (4)
- Infigratinib (1)
- Ingavirin (1)
- Ingenol (2)
- Inosine (2)
- Inositol (2)
- Intermediate (1)
- Intermedine (2)
- Iobitridol (1)
- Iodixanol (11)
- Iohexol (19)
- Iomeprol (3)
- Iopromide (11)
- Ioversol (8)
- Ipamorelin (1)
- ipidacrine (1)
- Ipragliflozin (11)
- Ipratropium (20)
- Iptacopan (9)
- Irbesartan (43)
- Irganox (14)
- Irinotecan (36)
- Isavuconazole (67)
- Iscotrizinol (7)
- Isocarboxazid (2)
- Isoconazole (7)
- Isocycloseram (1)
- Isoflurane (7)
- Isomalt (4)
- Isomaltitol (1)
- Isometheptene (13)
- Isoniazide (17)
- Isopropyl Palmitate (1)
- Isoproterenol (17)
- Isopulegol (2)
- Isosorbide (7)
- Isosorbide Mononitrate (9)
- Isosulfan Blue (5)
- Isothipendyl (6)
- Isotretinoin (39)
- Isoxsuprine (3)
- Isradipine (1)
- Istradefylline (7)
- Itraconazole (62)
- Ivabradine (83)
- Ivacaftor (33)
- Ivermectin (26)
- Ivosidenib (21)
- Ixabepilone (5)
- Ixazomib (15)
- Izuforant (3)
- Jacobine (1)
- Josamycin (11)
- Josamycin Propionate (6)
- Justicidin (8)
- Kaempferol (6)
- Kanamycin Sulfate (1)
- Ketamine Hydrochloride (16)
- Ketobemidone (5)
- Ketoconazole (46)
- ketoprofen (47)
- Ketorolac (43)
- Ketotifen Fumarate (20)
- Labetalol (38)
- Lacidipine (5)
- Lacosamide (52)
- Lactitol (6)
- Lactobionic (1)
- Lactofen (1)
- Lactoferrin (1)
- Lactulose (10)
- Lafutidine (19)
- Laidlomycin (1)
- Lamivudine (44)
- Lamotrigin (37)
- Landiolol (3)
- Laninamivir (5)
- Lanreotide (19)
- Lansoprazole (44)
- Lapatinib (30)
- Larotrectinib (20)
- Latanoprost (37)
- Lauramine Oxide (1)
- Lauroside (5)
- Lecirelin (1)
- Ledipasvir (31)
- Leflunomide (19)
- Lemborexant (10)
- Leminoprazole (1)
- Lenalidomide (76)
- Leniolisib (13)
- Lenvatinib (38)
- Lercanidiate (49)
- Lercanidipine (2)
- Lesinurad (8)
- Letermovir (3)
- Letrozole (20)
- Leucine (36)
- Leuprolide (6)
- Levacetylleucine (1)
- Levalbuterol (2)
- Levamisole (12)
- Levetiracetam (2)
- Levocarnitine (15)
- Levocetirizine (33)
- Levocloperastine (4)
- Levodopa (17)
- Levodropropizine (9)
- Levofloxacin (31)
- Levofolinate (12)
- Levomefolate (1)
- Levomepromazine (18)
- Levophanol (6)
- Levorgestrel (35)
- Levosimendan (17)
- Levosulpiride (9)
- Levothyroxine (67)
- Lidocaine (41)
- Liftegrast (22)
- Limaprost (2)
- Limonene (1)
- Linaclotide (15)
- Linagliptin (140)
- Linaprazan (4)
- Lincomycin (16)
- Linezolid (36)
- Linolenic (5)
- Liothyronine (12)
- Liraglutide (41)
- Lisdexamfetamine (2)
- Lisdexamphetamine (28)
- Lisinopril (20)
- Lobeglitazone (9)
- Lofepramine (2)
- Lofexidine (8)
- Lomefloxacin (1)
- Lomitapide (3)
- Lomustine (10)
- Lonafarnib (8)
- Lopamidol (16)
- Loperamide (20)
- Lopinavir (42)
- Loprazolam (8)
- Loratadine (49)
- Lorazepam (19)
- Lorcaserin (11)
- Lorlatinib (29)
- Lornoxicam (23)
- Losartan (66)
- Loteprednol (24)
- Lotilaner (9)
- Lovastatin (32)
- Loxapine (7)
- Loxoprofen (44)
- Lufenuron (9)
- Luliconazole (12)
- Lumacaftor (10)
- Lumateperone (66)
- Lupeol (1)
- Lurasidone (81)
- Lurbinectedin (8)
- Lusurrombopag (5)
- Luteolin (5)
- Lycopsamine (2)
- Lycorine (1)
- Lymecycline (7)
- Lynestrenol (4)
- Lypressin (1)
- Lysergide (1)
- Lysine (11)
- Macimorelin Acetate (1)
- Macitentan (43)
- Maduramicin (1)
- Malathion (6)
- Maltitol (3)
- Mancozeb (1)
- Mangiferin (1)
- Mangostin (1)
- Manidipine (20)
- Mannitol (5)
- Mapenterol (2)
- Maprotiline Hydrochloride (7)
- Maralixibat (2)
- Maraviroc (25)
- Marbofloxacin (8)
- Maribavir (9)
- Maropitant (9)
- Matairesinol (1)
- Mavacamten (23)
- Mavoglurant (2)
- Mavorixafor (33)
- Maytansine (6)
- Mebendazole (14)
- Mebeverine (30)
- Mecamylamine (3)
- Meclizine (23)
- Meclocycline (2)
- Meclofenamic Acid (6)
- Medetomidine (35)
- Medicarpin (1)
- Mefenamic Acid (15)
- Mefloquine (4)
- Megestrol Acetate (15)
- Meglumine (4)
- Melatonin (1)
- Meldonium (8)
- Melitracen (1)
- Meloxicam (19)
- Melperone (1)
- Melphalan (47)
- Melphalan Flufenamide (1)
- Memantine (18)
- Menadione (1)
- Menaquinone (3)
- Menbutone (4)
- Menthol (7)
- Menthone (7)
- Mepartricin (1)
- Mephenesin (2)
- Mephentermine (2)
- Mephenytoin (1)
- Mepivacaine (6)
- Meprednisone (2)
- Mepyramine Maleate (8)
- Mequitazine (1)
- Mercaptopurine (15)
- Meropenem (12)
- Mertansine (6)
- Mesalazine (56)
- Mesna (10)
- Mesosulfuron (3)
- Mesotrione (5)
- Mesterolone (5)
- Metacresol (14)
- Metamizole (23)
- Metaraminol (9)
- Metaxalone (20)
- Metconazole (15)
- Metergoline (1)
- Metformin (21)
- Methacholine Chloride (1)
- Methacycline (2)
- Methadone (6)
- Methamphetamine (1)
- Methazolamide (8)
- Methcathinone (2)
- Methenamine (11)
- Methenolone Acetate (1)
- Methionine (13)
- Methisoprinol (1)
- Methocarbamol (4)
- Methohexital (1)
- Methomyl (1)
- Methotrexate (49)
- Methoxsalen (3)
- Methsuximide (3)
- Methyclothiazide (1)
- Methyl Prednisolone Suleptanate (5)
- Methyl Salicylate (12)
- Methyldopa (15)
- Methylene Blue (19)
- Methylergometrine (10)
- Methylphenidate (22)
- Methylphenobarbital (1)
- Methylprednisolone (64)
- Metoclopramide (23)
- Metomidate (1)
- Metopimazine (4)
- Metoprolol (57)
- Metribuzin (1)
- Metronidazole (25)
- Metyrosine (2)
- Mevastatin (2)
- Mexazolam (3)
- Mexiletine (12)
- Mianserin (7)
- Micafungin (10)
- Miconazole (16)
- Midazolam (35)
- Midodrine Hydrochloride (21)
- Midostaurin (19)
- Mifepristone (25)
- Migalastat (18)
- Miglitol (1)
- Miglustat (19)
- Milbemycin (12)
- Milnacipran (20)
- Milrinone (20)
- Miltefosine (8)
- Minocycline (41)
- Minoxidil (15)
- Mirabegron (109)
- Miramistin (1)
- Miriplatin (3)
- Mirogabalin (21)
- Mirtazapine (29)
- Miscellaneous (444)
- Miscellaneus (4)
- Misoprostol (13)
- Mitapivat (20)
- Mitomycin (19)
- Mitotane (3)
- Mitoxantrone (7)
- Mivacurium (24)
- Mizolastine (1)
- Mobocertinib (10)
- Moclobemide (3)
- Modafinil (17)
- Moexipril (14)
- Molindone (9)
- Molnupiravir (22)
- Molsidomine (9)
- Momelotinib (11)
- Mometasone (36)
- Monensin (1)
- Monepantel (2)
- Monocrotaline (2)
- Montelukast (72)
- Morantel (6)
- Morniflumate (2)
- Mosapride (16)
- Motixafortide (1)
- Moxidectin (19)
- Moxifloxacin (54)
- Moxisylyte (1)
- Moxonidine (15)
- Mupirocin (25)
- Mutilin (2)
- Mycophenolate (50)
- Myristic Acid (1)
- N-nitroso coco methylamine (1)
- Nabilone (10)
- Nabumetone (15)
- Nadifloxacin (2)
- Nadolol (17)
- Nafcillin (1)
- Naftazone (1)
- Naftidrofuryl Oxalate (7)
- Naftifine (5)
- Naftopidil (13)
- Naldemedine (3)
- Nalfurafine (2)
- Nalidixic Acid (2)
- Nalmefene (4)
- Naloxegol (3)
- Naloxegol D5 (1)
- Naloxon (31)
- Naltrexone (34)
- Nandrolone Decanoate (14)
- Naphazoline (8)
- Naproxen (51)
- Narasin (1)
- Naratriptan (26)
- Naringenin (2)
- Narirutin (2)
- Natamycin (1)
- Nateglinide (15)
- Neamine (3)
- Nebivolol (101)
- Nebramine (2)
- Nebrosamine (3)
- Nedocromil (5)
- Nefopam (12)
- Nelarabine (27)
- Neohesperidin Dihydrochalcone (8)
- Neomycin Sulfate (24)
- Neostigmine (15)
- Neotame (5)
- Neoxanthin (1)
- Nepafenac (13)
- Neratinib (10)
- Netarsudil (25)
- Netilmicin (5)
- Nevirapine (14)
- Niacin (16)
- Nicametate (1)
- Nicarbazine (1)
- Nicardipine (28)
- Nicergoline (14)
- Niclosamide (6)
- Nicorandil (17)
- Nicotinamide (7)
- Nicotine (45)
- Nicotinic Acid (12)
- Nifedipine (17)
- Niflumic Acid (6)
- Nifuratel (18)
- Nifuroxazide (6)
- Nifursol (2)
- Nifurtimox (1)
- Nilotinib (66)
- Nimesulide (12)
- Nimodipine (16)
- Nimorazole (4)
- Nintedanib (80)
- Niranthin (1)
- Niraparib (71)
- Nirmatrelvir (48)
- Nirogacestat (7)
- Nisoldipine (8)
- Nitazoxanide (7)
- Nitenpyram (6)
- Nitisinone (5)
- Nitrazepam (7)
- Nitrendipine (5)
- Nitrilotriacetic Acid (2)
- Nitrofural (4)
- Nitrofurantoin (10)
- Nitroscanate (1)
- Nitroxynil (2)
- Nizatidine (21)
- Nomegestrol (7)
- Noradrenaline (12)
- Noradrenolone (1)
- Norbelladine (3)
- Norelgestromin (3)
- Norepinephrine (41)
- Norethindrone (30)
- Norethindrone Acetate (26)
- Norfloxacin (20)
- Norgestimate (8)
- Norgestrel (16)
- Nortriptyline (24)
- Noscapine (4)
- Novaluron (6)
- Nylidrin (1)
- Nystatin (8)
- Obeticholic Acid (29)
- Oclacitinib (2)
- Octenidine (3)
- Octinoxate (1)
- Octisalate (5)
- Octocrylene (2)
- Octreotide (36)
- Ofloxacin (18)
- Olanzapine (47)
- Olaparib (45)
- Oleic Acid (5)
- Oliceridine (3)
- Olmesartan (99)
- Olodaterol (2)
- Olopatadine (27)
- Olsalazine (10)
- Olutasidenib (1)
- Omadacycline (12)
- Omeprazole (91)
- Omidenepag Isopropyl (1)
- Ondansetron (30)
- Opicapone (2)
- Opipramol (2)
- Orbifloxacin (9)
- Orciprenaline (5)
- Orientin (1)
- Oritavancin (23)
- Orlistat (33)
- Ormeloxifene (5)
- Ornidazole (8)
- Ornithine (18)
- Orotic Acid (3)
- Orphenadrine (20)
- Oseltamivir (83)
- Osilodrostat (1)
- Osimertinib (39)
- Ospemifene (2)
- Oteracil (1)
- Oteseconazole (2)
- Otilonium Bromide (6)
- Oxacillin (11)
- Oxaliplatin (11)
- Oxandrolone (4)
- Oxaprozin (8)
- Oxazepam (6)
- Oxcarbazepine (44)
- Oxeladin (5)
- Oxethazaine (1)
- Oxetorone (3)
- Oxfendazole (4)
- Oxiracetam (2)
- Oxitropium (6)
- Oxolamine (2)
- Oxomemazine (6)
- Oxprenolol (1)
- Oxybenzone (1)
- Oxybutynin (28)
- Oxychlordane (1)
- Oxyclozanide (1)
- Oxycodone (10)
- Oxymetazoline (7)
- Oxymetholone (2)
- Oxymorphone (4)
- Oxyphenbutazone (1)
- Oxyphencyclimine (1)
- Oxyquinoline (2)
- Oxytetracycline (14)
- Oxytocin (21)
- Ozanimod (15)
- Ozenoxacin (5)
- Paclitaxel (176)
- Pacritinib (3)
- Padimate O (2)
- Pafolacianine (2)
- Palbociclib (124)
- Paliperidone (52)
- Palonosetron (39)
- Palovarotene (1)
- Pamabrom (1)
- Pamidronate (5)
- Pamoic Acid (1)
- Pancuronium Bromide (6)
- Panobinostat (1)
- Pantoprazole (80)
- Pantothenic Acid (9)
- Papaverine Hydrochloride (9)
- Paraben (14)
- Paracetamol (36)
- Parbendazole (1)
- Parecoxib (33)
- Pargeverine (4)
- Paricalcitol (5)
- Paromomycin (1)
- Parthenolide (1)
- Pasireotide (6)
- Pazopanib (45)
- Pefloxacin (9)
- Pelubiprofen (2)
- Pemafibrate (4)
- Pembrolizumab (1)
- Pemetrexed (65)
- Pemigatinib (6)
- Penciclovir (29)
- Pendimethalin (7)
- Penicillamine (16)
- Penicillin (67)
- Penoxsulam (2)
- Pentagastrin (1)
- Pentamidine (7)
- Pentazocine (1)
- Pentetreotide (1)
- Pentobarbital (6)
- Pentoxifylline (23)
- Peramivir (6)
- Perampanel (20)
- Perazine (1)
- Perazine Dimalonate (1)
- Pergolide (18)
- Perhexiline (2)
- Pericyazine (1)
- Perindopril (47)
- Permethrin (37)
- Perospirone (6)
- Perphenazine (8)
- Pethidine (12)
- Pharacine (4)
- Phenacetin (2)
- Phenazepam (1)
- Phenazopyridine (4)
- Phenelzine (2)
- Pheniramine (7)
- Phenol (12)
- Phenoxybenzamine (11)
- Phenoxyethanol (2)
- Phentermine (7)
- Phentolamine (6)
- Phenylalanine (12)
- Phenylbutazone (9)
- Phenylbutyrate (12)
- Phenylephrine (84)
- Phenylpropanolamine (6)
- Phenyltoloxamine (2)
- Phenyramidol (2)
- Phenytoin (12)
- Phloroglucinol (14)
- Pholcodine (7)
- Phthalylsulfathiazole (1)
- Phytonadione (71)
- Pibrentasvir (5)
- Picaridin (1)
- Picloram (2)
- Picloxydine (1)
- Picosulfate Sodium (8)
- Pidotimod (20)
- Pilocarpine (12)
- Pimavanserin (32)
- Pimecrolimus (11)
- Pimobendan (6)
- Pimozide (7)
- Pinaverium (18)
- Pindolol (6)
- Pinoxaden (1)
- Pioglitazone (39)
- Pipamperone (3)
- Piperacillin (33)
- Piperaquine (8)
- Piperidine (15)
- Pipotiazine (1)
- Pirarubicin (4)
- Pirenzepine (5)
- Pirfenidone (31)
- Piribedil (5)
- Piritramide (2)
- Pirlimycin (1)
- Piroxicam (17)
- Pirtobrutinib (2)
- Pitavastatin (66)
- Pitofenone (10)
- Pitolisant (24)
- Pivmecillinam (10)
- Pizotifen (1)
- Plazomicin (12)
- Plecanatide (1)
- Plerixafor (40)
- Polmacoxib (10)
- Polyhexanide (2)
- Polymyxin B (17)
- Polythiazide (2)
- Pomalidomide (40)
- Ponatinib (13)
- Ponesimod (4)
- Porfiromycin (1)
- Posaconazole (150)
- Pralatrexate (14)
- Pralidoxime (3)
- Pralmorelin (2)
- Pralsetinib (6)
- Pramipexole (58)
- Pramoxine (1)
- Pranlukast (3)
- Prasugrel (69)
- Pravastatin (22)
- Prazepam (4)
- Praziquantel (20)
- Prazosin (14)
- Prednicarbate (8)
- Prednisolone (93)
- Prednisolone Pivalate (1)
- Prednisolone Sodium Phosphate (2)
- Prednisone (23)
- Pregabalin (89)
- Pregnenolone (2)
- Pretomanid (21)
- Pridinol (4)
- Prilocaine (24)
- Primapterin (4)
- Primaquine (8)
- Primidone (8)
- Probenecid (7)
- Procainamide (2)
- Procaterol (2)
- Prochlorperazine (32)
- Procyanidin (3)
- Procyclidine (2)
- Progesterone (93)
- Proguanil (8)
- Promazine (11)
- Promestriene (3)
- Promethazine (22)
- Propafenone (22)
- Propamidine (1)
- Propantheline Bromide (2)
- Proparacaine (13)
- Propentofylline (1)
- Propiconazole (4)
- Propiomazin (1)
- Propiverine (1)
- Propofol (42)
- Propoxur (1)
- Propranolol (35)
- Propyl Gallate (4)
- Propylhexedrine (2)
- Propylthiouracil (3)
- Prothioconazole (2)
- Protriptyline (2)
- Prucalopride (45)
- Prulifloxacin (9)
- Pterostilbene (1)
- Pulegone (1)
- Pyraclostrobin (3)
- Pyrantel (7)
- Pyrazinamide (4)
- Pyridine (9)
- Pyridostigmine (5)
- Pyridoxal (4)
- Pyridoxamine (2)
- Pyridoxine (35)
- Pyrimethamine (15)
- Pyronaridine (10)
- Pyroxasulfone (2)
- Pyrvinium Pamoate (5)
- Quercetin (7)
- Quetiapine (74)
- Queuine (1)
- Quinalizarin (1)
- Quinapril (17)
- Quinapyramine (2)
- Quinfamide (1)
- Quinidine (11)
- Quinine (9)
- Quinoline (5)
- Quinupristin (1)
- Quizartinib (1)
- Rabeprazole (1)
- Racecadotril (13)
- Ractopamine (4)
- Rafoxanide (1)
- Raloxifene (51)
- Raltegravir (35)
- Ramelteon (46)
- Ramipril (45)
- Ramosetron (6)
- Ranitidine (20)
- Ranolazine (50)
- Rapamycin (43)
- Rapidosept (9)
- Rasagiline (40)
- Ravuconazole (6)
- Rebamipide (10)
- Reboxetine (1)
- Regadenoson (16)
- Regorafenib (38)
- Rehmaionoside (3)
- Relebactam (3)
- Relugolix (67)
- Remdesivir (57)
- Remifentanil (15)
- Remimazolam (9)
- Remogliflozin (14)
- Repaglinide (24)
- Repotrectinib (1)
- Reproterol (5)
- Resmetirom (28)
- Resocortol (1)
- Resveratrol (3)
- Retinol (18)
- Retinyl palmitate (2)
- Retrorsine (2)
- Revefenacin (50)
- Revumenib (11)
- Rezafungin (1)
- Rhodamine (2)
- Ribavirin (9)
- Ribociclib (66)
- Riboflavin (19)
- Ridinilazole (6)
- Rifabutin (13)
- Rifampicin (26)
- Rifapentine (10)
- Rifaximin (18)
- Rigosertib (10)
- Rilpivirine (34)
- Riluzole (13)
- Rimegepant (32)
- Riociguat (52)
- Ripasudil (7)
- Ripretinib (5)
- Risdiplam (8)
- Risedronic Acid (12)
- Risperidone (50)
- Ritlecitinib (25)
- Ritonavir (73)
- Rivaroxaban (156)
- Rivastigmine (37)
- Rizatriptan (34)
- Robenacixib (12)
- Rocuronium (19)
- Roflumilast (37)
- Rolapitant (2)
- Romidepsin (14)
- Romifidine hydrochloride (1)
- Ropinirole (49)
- Ropivacaine (14)
- Rosavin (1)
- Rosiglitazone (3)
- Rosuvastatin (5)
- Rotigotine (21)
- Roxadustat (45)
- Roxatidine (3)
- Roxithromycin (15)
- Rubber Oligomers (17)
- Rucaparib (22)
- Rufinamide (14)
- Rupatadine (22)
- Rutin (9)
- Ruxolitinib (2)
- Sabacic Acid (1)
- Saccharin (1)
- Safinamide (23)
- Saflufenacil (4)
- Salacinol (1)
- Salbutamol (79)
- Salcaprozate (5)
- Salidroside (1)
- Salinomycin (1)
- Salmeterol (35)
- Samidorphan (9)
- Sapropterin (43)
- Saquinavir (9)
- Sarecycline (2)
- Saroglitazar (5)
- Satranidazole (1)
- Savarexant (29)
- Saviprazole (1)
- Saxagliptin (101)
- Scopolamine (7)
- Scopoletin (1)
- Secnidazole (5)
- Seladelpar (5)
- Selamectin (5)
- Selegiline (12)
- Selexipag (28)
- Selinexor (13)
- Selpercatinib (40)
- Seltorexant (1)
- Selumetinib (30)
- Semaglutide (41)
- Semduramicin (2)
- Senecionine (2)
- Seneciphylline (2)
- Senecivernine (2)
- Senkirkine (1)
- Sennoside (7)
- Serotonin (5)
- Sertaconazole (3)
- Sertraline (52)
- Setmelanotide (2)
- Sevoflurane (9)
- shogaol (1)
- Sibutramine (25)
- Silibinin (9)
- Silodosin (49)
- Simethicone (6)
- Sincalide (2)
- Siponimod (74)
- Sitafloxacin (4)
- Sitagliptin (157)
- Sitravatinib (1)
- Sivelestat (10)
- Slidenafil (55)
- Sobrerol (5)
- Sodium stearyl fumarate (9)
- Sofosbuvir (129)
- Sofpironium Bromide (15)
- Solifenacin (55)
- Solriamfetol (20)
- Somatostatin (5)
- Sonidegib (2)
- Sorafenib (57)
- Sorbic Acid (2)
- Sorbitol (4)
- Sotagliflozin (19)
- Sotalol (9)
- Sotorasib (8)
- Sovateltide (2)
- Sparfloxacin (1)
- Sparsentan (14)
- Spectinomycin (9)
- Spilanthol (2)
- Spinosyn (17)
- Spiramycin (11)
- Spironolactone (30)
- Squalene (1)
- Stanozolol (3)
- Stevioside (1)
- Stigmastanol (7)
- Stiripentol (14)
- Strychnine (1)
- Succinylcholine (3)
- Sucralfate (7)
- Sucralose (9)
- Sufentanil (9)
- Sugammadex (88)
- Sulbactam (10)
- Sulbatiamine (1)
- Sulfacetamide (5)
- Sulfachloropyridazine (1)
- Sulfadiazine (11)
- Sulfadimethoxine (7)
- Sulfadoxine (10)
- Sulfaguanidine (3)
- Sulfalene (2)
- Sulfamerazine (1)
- Sulfamethazine (1)
- Sulfamethoxazole (15)
- Sulfametomidine (1)
- Sulfaphenazole (1)
- Sulfapyride (13)
- Sulfaquinoxaline (1)
- Sulfasalazine (19)
- sulfathiazole (1)
- Sulindac (5)
- Sulisobenzone (1)
- Sulphanilamide (5)
- Sultamicillin (9)
- Sumatriptan (41)
- Sunitinib (31)
- Suvorexant (2)
- Tacrolimus (40)
- Tadalafil (49)
- Tafamidis (20)
- Tafenoquine (9)
- Tafluprost (9)
- Talampicillin (1)
- Talazoparib (2)
- Tamoxifen (33)
- Tamsulosin (61)
- Tandospirone (4)
- Tanespimycin (1)
- Tapentadol (19)
- Tapinarof (18)
- Tartrazine (5)
- Tasimelteon (18)
- Taurine (1)
- Taurodeoxycholic Acid (2)
- Taurolidine (7)
- Taurolithocholic Acid (3)
- Tavaborole (17)
- Taxifolin (1)
- Tazarotene (16)
- Tazemetostat (12)
- Tazobactam (11)
- Tebipenem Pivoxil (7)
- Tebuconazole (1)
- Tecovirimat (2)
- Tedizolid (18)
- Teduglutide (3)
- Teferdin (1)
- Tegafur (1)
- Tegaserod (2)
- Tegoprazan (35)
- Teicoplanin (6)
- Telaprevir (4)
- Telavancin (5)
- Telbivudine (3)
- Telithromycin (2)
- Telmisartan (88)
- Telotristate Ethyl (38)
- Temazepam (9)
- Temocapril (2)
- Temozolomide (15)
- Temsirolimus (6)
- Teneligliptin (42)
- Tenofovir (153)
- Tenoxicam (14)
- Tepotinib (6)
- Teprenone (2)
- Terazosin (23)
- Terbinafine (31)
- Terbutaline (17)
- Terconazole (3)
- Terephthalic acid (15)
- Terfenadine (10)
- Teriflunomide (43)
- Teriparatide (3)
- Terlipressin (2)
- Ternidazole (3)
- Terpin (1)
- Tesofensine (3)
- Testosterone (116)
- Testosterone Cypionate (10)
- Testosterone Decanoate (7)
- Tetrabenazine (67)
- Tetracaine (31)
- Tetraconazole (1)
- Tetracycline (10)
- Tetraxetan (2)
- Tetrazepam (5)
- Tetryzoline Hydrochloride (8)
- Tezacaftor (11)
- Thalidomide (4)
- Thebaine (1)
- Theophylline (24)
- Thiamazole (7)
- Thiamethoxam (1)
- Thiamine (47)
- Thiamphenicol (2)
- Thimerosal (2)
- Thiocolchicoside (16)
- Thioctic Acid (23)
- Thioguanine (2)
- Thiopental (4)
- Thioridazine (8)
- Thiorphan (3)
- Thiotepa (9)
- Thiothixene (5)
- Thonzylamine (2)
- Threonine (9)
- Thymol (1)
- Thymosin (1)
- Tiagabine (19)
- Tiamulin (23)
- Tianeptine (8)
- Tiapride (5)
- Tibolone (12)
- Ticagrelor (269)
- Ticlopidine (14)
- Tiemonium Methylsulfate (1)
- Tigecycline (27)
- Tildipirosin (1)
- Tilidine (5)
- Tilmicosin (1)
- Tilorone (1)
- Timolol (38)
- Timoprazole (1)
- Tinidazole (10)
- Tioconazole (5)
- Tiopronin (17)
- Tiotropium (23)
- Tipepidine (1)
- Tipiracil (2)
- Tirbanibulin (6)
- Tirofiban (9)
- Tirzepatide (16)
- Tivozanib (9)
- Tizanidine (29)
- Tizoxanide (3)
- Tobramycin (14)
- Tocopherol (9)
- Tocopheryl acetate (8)
- Tofacitinib (122)
- Tofisopam (5)
- Tolbutamide (4)
- Tolcapone (9)
- Toldimfos (1)
- Tolfenamic acid (4)
- Tolimidone (1)
- Tolmetin (7)
- Tolnaftate (8)
- Tolperisone (7)
- Tolterodine (48)
- Toltrazuril (3)
- Tolvaptan (61)
- Tonibral (1)
- Topiramate (31)
- Topiroxostat (2)
- Topotecan (2)
- Torasemide (22)
- Toremifene (3)
- Tosylchloramide Sodium (1)
- Tovorafenib (8)
- Toxaphene (4)
- Trabectedin (16)
- Tramadol (19)
- Trametinib (32)
- Trandolapril (17)
- Tranexamic Acid (14)
- Tranylcypromine (8)
- Travoprost (14)
- Trazodone (42)
- Trehalose (3)
- Trelagliptin (12)
- Trenbolone Acetate (2)
- Treosulfan (2)
- Treprostinil (18)
- Tretinoin (10)
- Triamcinolone (11)
- Triamcinolone Acetonide (17)
- Triamcinolone Hexacetonide (4)
- Triamterene (7)
- Triazolam (2)
- Tribenoside (5)
- Trichlormethiazide (1)
- Trichodesmine (1)
- Triclabendazole (10)
- Triclocarban (3)
- Triclopyr (4)
- Triclosan (3)
- Trientine (36)
- Trifarotene (10)
- Triflumuron (1)
- Trifluoperazine (12)
- Trifluridine (6)
- Triflusal (5)
- Trigonelline (1)
- Triheptanoin (4)
- Trihexyphenidyl (4)
- Trilaciclib (9)
- Trilostane (10)
- Trimebutine (19)
- Trimetazidine (23)
- Trimethobenzamide (12)
- Trimethoprim (24)
- Trimipramine (11)
- Tripalmitin (2)
- Triprolidine (5)
- Triptorelin (15)
- Triricinolein (5)
- Trofinetide (17)
- Trolamine (4)
- Tropicamide (6)
- Tropisetron (3)
- Trospium (6)
- Troxerutin (16)
- Tryptophan (24)
- Tryptophol (5)
- Tucatini (22)
- Tulathromycin (14)
- Tulobuterol (1)
- Turpinionoside (5)
- Tylosin (2)
- Tyrothricin (1)
- Ubidecarenone (9)
- Ubrogepant (8)
- Ulinastatin (1)
- Ulipristal (21)
- Umbralisib (6)
- Umckalin (1)
- Umeclidinium Bromide (12)
- Umifenovir (8)
- Upadacitinib (23)
- Urapidil (23)
- Uridine (11)
- Ursodeoxycholic (35)
- Vaborbactam (26)
- Vadadustat (19)
- Valaciclovir (28)
- Valbenazine (16)
- Valdecoxib (2)
- Valerenic Acid (3)
- Valethamate Bromide (1)
- Valganciclovir (42)
- Validamycin (1)
- valine (1)
- Valproic Acid (40)
- Valsartan (6)
- Vamorolone (1)
- Vancomycin (28)
- Vandetanib (9)
- Vanzacaftor (1)
- Vardenafil (35)
- Varenicline (65)
- Varespladib (3)
- Vasopressin (28)
- Vecabrutinib (1)
- Vecuronium Bromide (8)
- Velpatasvir (57)
- Vemurafenib (1)
- Venetoclax (59)
- Venlafaxine (41)
- Verapamil (48)
- Vericiguat (12)
- Vestitol (1)
- Vibegron (58)
- Vicenin (1)
- Vidarabine (1)
- Vigabatrin (17)
- Vilanterol (40)
- Vilazodone (69)
- Vildagliptin (86)
- Viloxazine (20)
- Vinblastine Sulfate (11)
- Vinclozolin (1)
- Vincristine Sulfate (10)
- Vinflunine (7)
- Vinorelbine (18)
- Vinpocetine (6)
- Virginiamycin (3)
- Vismodegib (39)
- Vitexin (4)
- Voacamine (1)
- Voclosporin (13)
- Voglibose (11)
- Vonoprazan (76)
- Vorapaxar (1)
- Vorasidenib (7)
- Voriconazole (44)
- Vorinostat (1)
- Vortioxetine (67)
- Vosoritide (20)
- Voxelotor (2)
- Warburganal (1)
- Warfarin (13)
- Xanomeline (8)
- Xanthine (1)
- Xanthinol (1)
- Xanthone (2)
- Xipamide (4)
- Xylazine (6)
- Xylometazoline (7)
- Yohimbine (5)
- Zafirlukast (22)
- Zaleplon (6)
- Zaltoprofen (2)
- Zalunfiban (2)
- Zanamivir (8)
- Zanubrutinib (26)
- Zastaprazan (1)
- Zavegepant (8)
- Zearalenone (2)
- Zeaxanthin (2)
- Zephirol (7)
- Zidovudine (20)
- Zileuton (14)
- Zilpaterol (2)
- Ziprasidone (43)
- Ziram (1)
- Zofenopril (13)
- Zoledronic Acid (18)
- Zolmitriptan (38)
- Zolpidem (22)
- Zonisamide (10)
- Zopiclone (29)
- Zotatifin (1)
- Zotepine (3)
- Zoxamide (1)
- Zuclopenthixol (1)
- Zuclopenthixol Decanoate (3)
- Zuranolone (1)
- ImpurityStandards (907)
- Afoxolaner (1)
- Astaxanthin (1)
- cafedrine (3)
- Camostat (2)
- Campestanol (1)
- Capivasertib (25)
- Caricaxanthin (1)
- Carteolol (11)
- Cebranopadol (10)
- Cefodizime (2)
- Cefotetan (2)
- Cefovecin (1)
- Cefquinome (1)
- Chiglitazar (2)
- Chlorantraniliprole (14)
- Chloroprocaine (11)
- Chloropyramine (1)
- Cimaterol (2)
- Cimbuterol (1)
- Clazuril (10)
- Clenpenterol (1)
- Clerosterol (1)
- Clethodim (7)
- Clofedanol (2)
- Clotiazepam (2)
- Cyphenothrin (1)
- Cyproconazole (1)
- Daprodustat (16)
- Diclazuril (4)
- Erdosteine (2)
- Estazolam (1)
- Exenatide (2)
- Fenvalerate (3)
- Fosaprepitant (28)
- Fulvestrant (55)
- Gemifloxacin (7)
- Gepirone (9)
- Gramicidin (6)
- Leuprolide (25)
- Meradimate (1)
- Miscellaneous (1)
- Novobiocin (2)
- Odevixibat (6)
- Omaveloxolone (1)
- Omethoate (1)
- Osunprotafib (2)
- retapamulin (7)
- Revefenacin (1)
- Rifamycin (13)
- Ruxolitinib (6)
- Sacubitril (97)
- sarpogrelate (8)
- Teriparatide (5)
- Thymalfasin (1)
- Tirzepatide (22)
- Valsartan (48)
- Lercanidipine (1)
- Paroxetine (2)
Sort Product
'Lenacapavir' related Reference Standards & Products
Showing 55–63 of 63 results
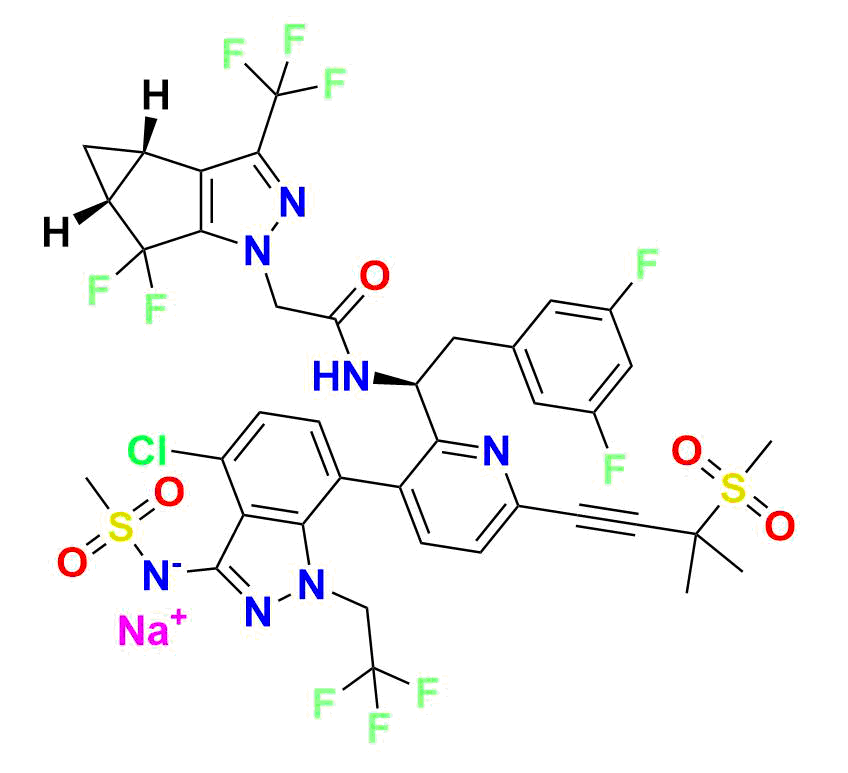
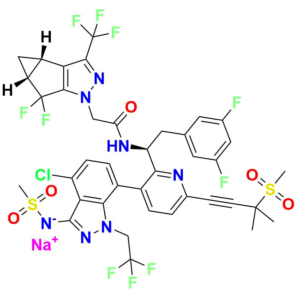
As a supplier of high-purity analytical standards, we offer a comprehensive portfolio of Lenacapavir impurities designed for pharmaceutical retailers, API manufacturers, and regulatory bodies. Our products support every stage of drug development and quality assurance—from forced-degradation profiling to method validation and stability studies.

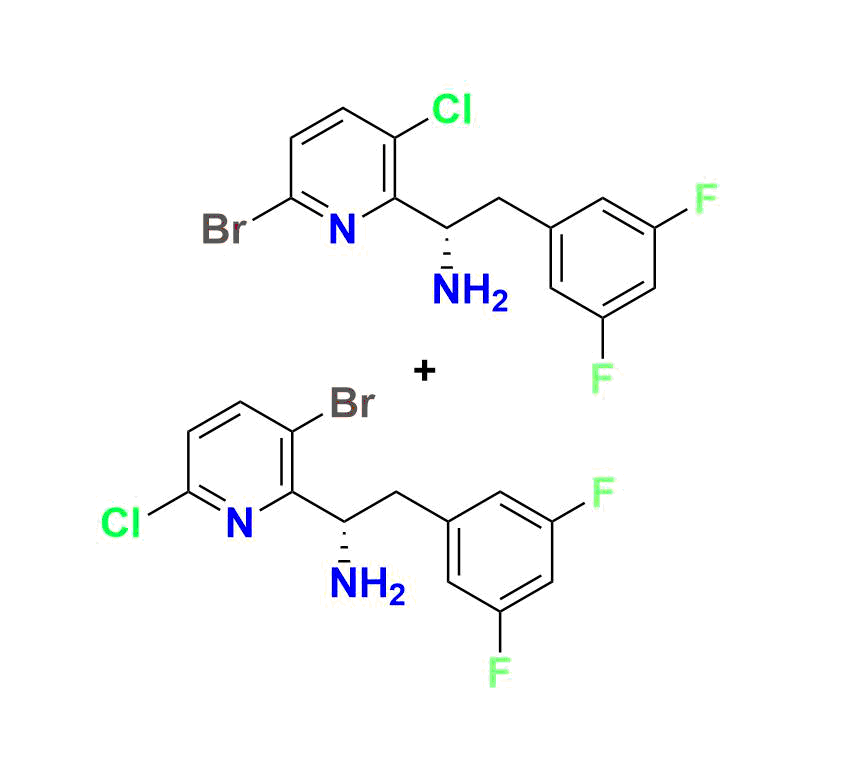
Lenacapavir Impurity 1 (CAS 2189680‑65‑5):
Low-molecular-weight degradation fragment (~282 g/mol; molecular formula C₁₀H₇F₅N₂O₂), ideal for calibration of early degradation peaks in HPLC/LC‑MS assays
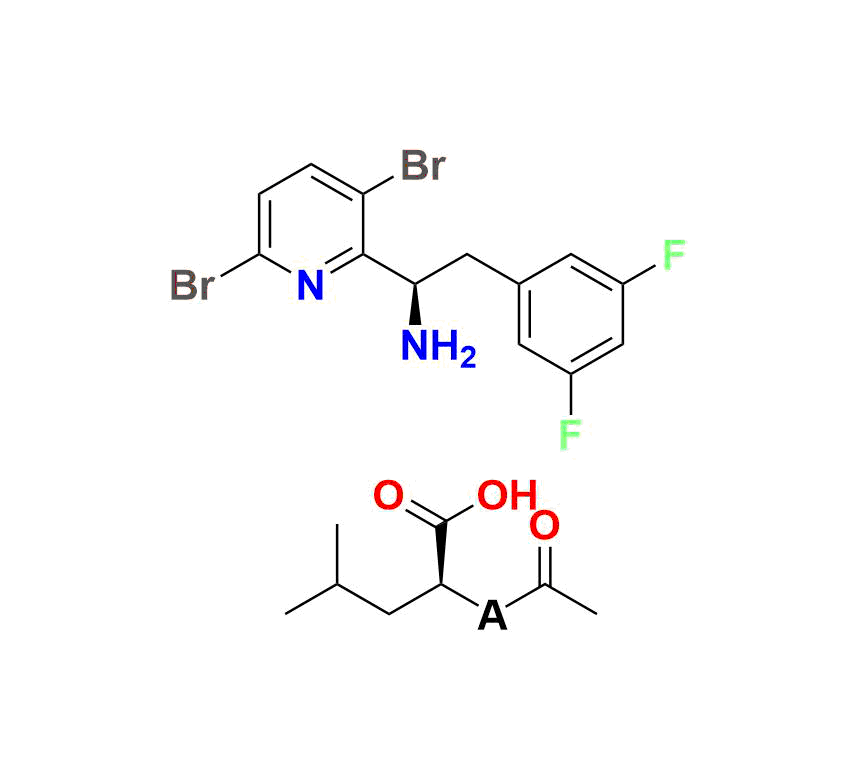
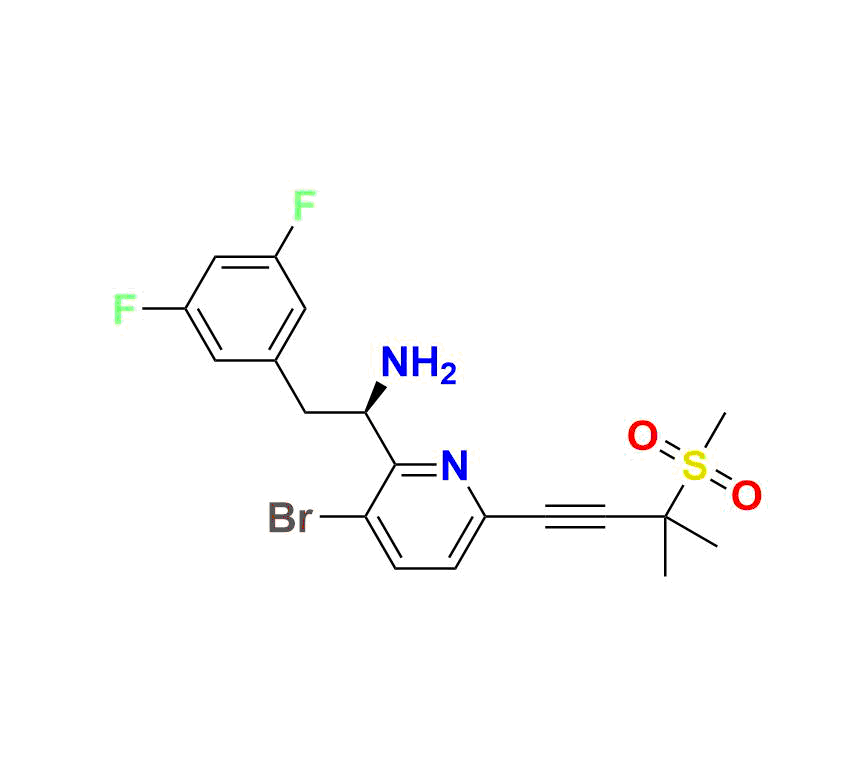
Lenacapavir Impurity 7:
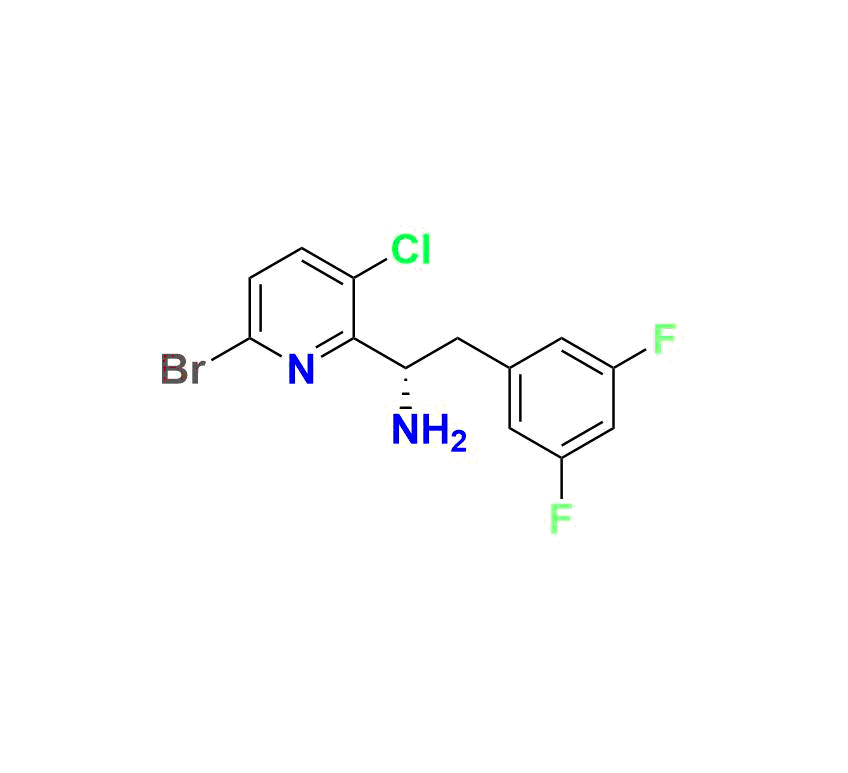
(R)-1 (3‑bromo‑6‑(3‑methyl‑3‑(methylsulfonyl)but‑1‑yn‑1‑yl)pyridin‑2‑yl)-2‑(3,5‑difluorophenyl)ethan-1‑amine, MW ~457.3 g/mol—commonly used to monitor forced‑degradation products and validate stability testing
Lenacapavir Impurity 9:
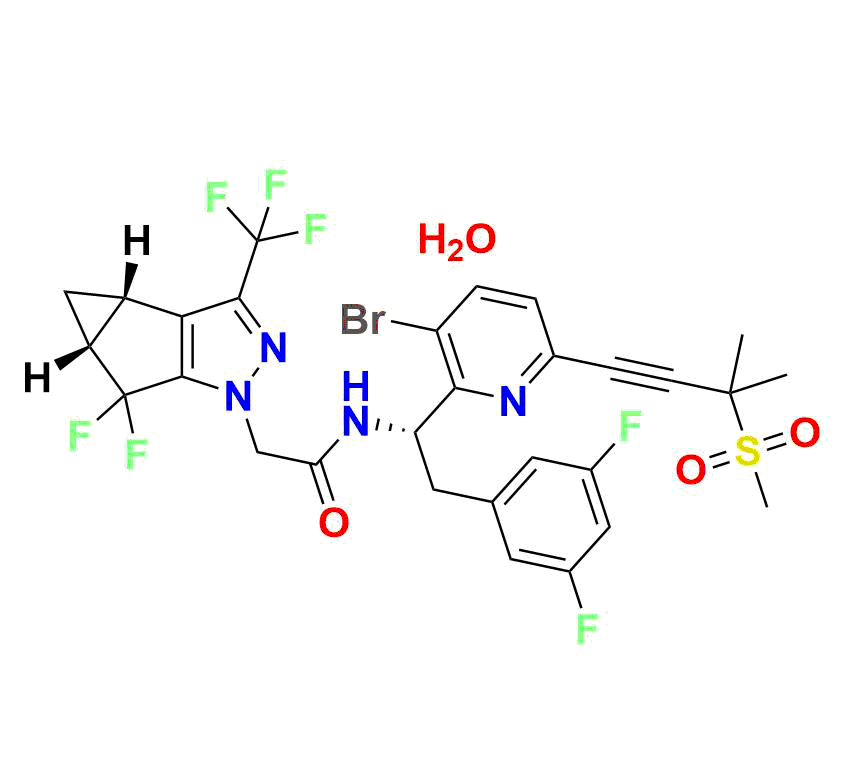
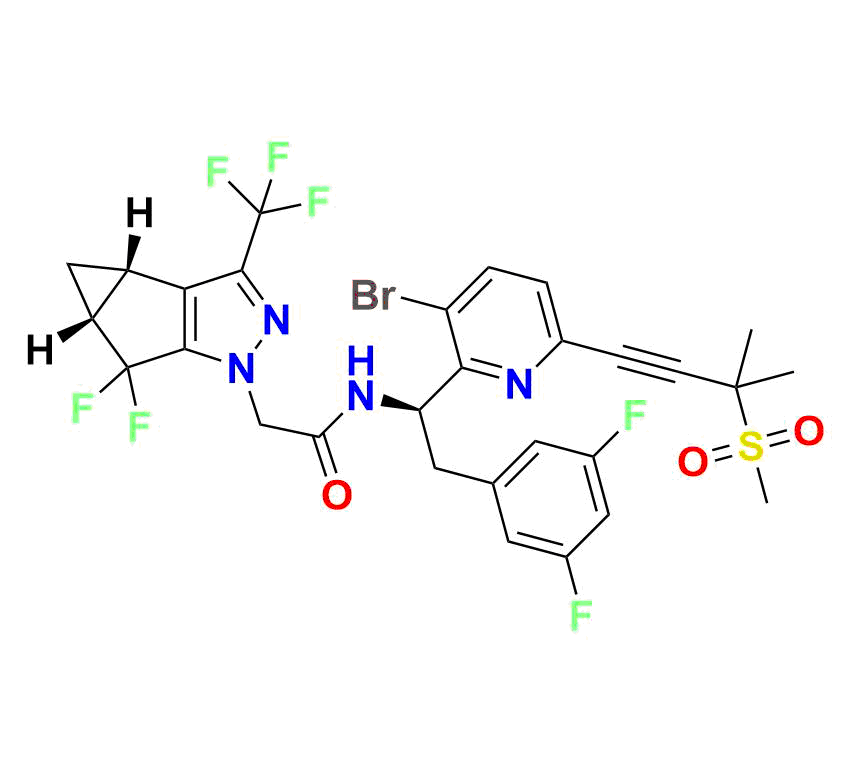
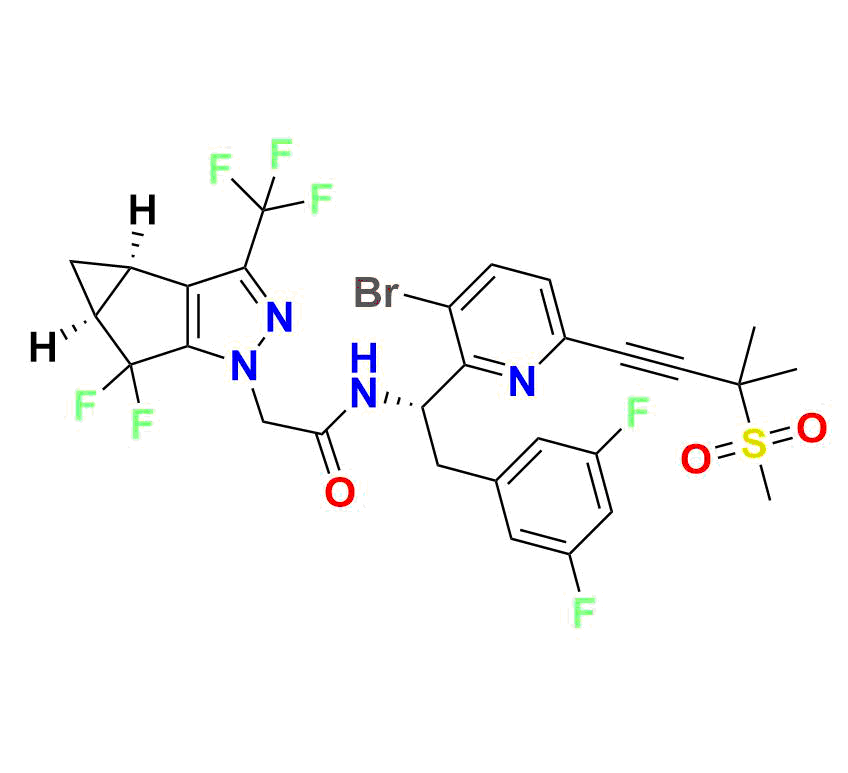
High‑molecular-weight impurity (~721.5 g/mol; molecular formula C₂₉H₂₄BrF₇N₄O₃S), often encountered as a late-stage synthetic impurity or degradation marker
Other Available Standards:
Including Impurities 4, 5, and 60—representing bromopyridine intermediates, process markers, and custom impurities with detailed char-acterization data

Regulatory-Grade Quality:
Each impurity standard is supplied with a Certificate of Analysis (CoA), stability data, and traceability to pharmacopeial reference or internal benchmarks to support DMF, ANDA, NDA approvals or stability submissions
Full Characterization:
Provided technical documentation includes purity analysis, structural confirmation (NMR, MS), and retention behavior—crucial for reliable method development and validation
Analytical & Method Validation Utility:
Ideal for forced-degradation studies, impurity profiling, and QC testing of Lenacapavir API or formulated dosage forms
Custom & Isotopically Labeled Compounds:
Capabilities include traceable isotopes (e.g., ^13C/^2H₃‑Lenacapavir), metabolites, and nitrosamine impurity analogs, based on customer specifications
Compliance with ICH Q3A/Q3B Guidelines:
Supports global regulatory alignment by providing reference standards for specified and unspecified impurities, aiding impurity limits documentation and validation.