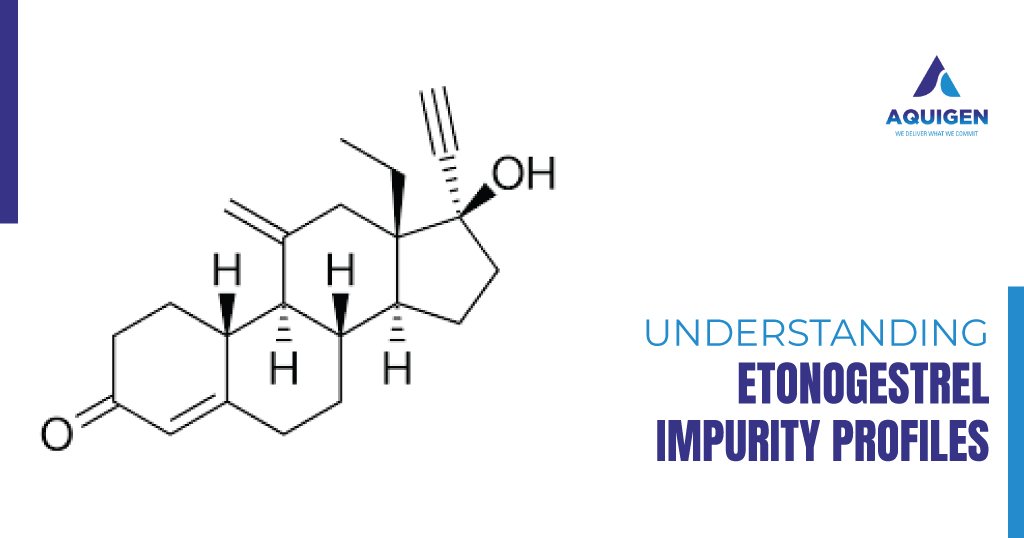
Etonogestrel, a third-generation progestin, has become a cornerstone ingredient in hormonal contraceptives and other related drug formulations, owing to its efficacy and safety profile. However, like any active pharmaceutical ingredient (API), the presence of impurities in etonogestrel can affect both the efficacy and safety of the final drug products. Impurity profiling is crucial, as it provides insights into the stability, toxicity, and overall quality of the drug. This process plays a pivotal role in meeting stringent regulatory requirements and ensuring that medicines are both effective and safe for consumers.
At Aquigen Bio Sciences, we take pride in being a leading provider of etonogestrel impurities standards in India. With a strong commitment to quality and innovation, we support pharmaceutical companies in thorough impurity profiling to ensure compliance with global regulatory standards. Let’s delve into the importance of understanding etonogestrel impurity profiles and how it impacts hormonal drug development.
What Are Etonogestrel Impurities?
Etonogestrel impurities refer to unintended chemical substances that exist within the API or the final drug product. The presence of impurities, even in trace amounts, can influence the drug’s safety and efficacy. Therefore, their identification, quantification, and regulation are integral components of drug development. These impurities can arise from various sources during the manufacturing process, such as:
- Raw materials: Impurities in the starting materials may end up in the final product.
- Synthetic pathways: By-products formed during chemical reactions.
- Degradation products: Breakdown of etonogestrel due to environmental factors like heat, light, or moisture.
- Storage and packaging complexities: Interaction with packaging materials or exposure to adverse conditions.
Regulatory Importance of Impurity Profiling in Hormonal Drugs
Regulatory bodies such as the ICH, the U.S. FDA, and the European Medicines Agency (EMA) place significant emphasis on impurity profiling. Guidelines like ICH Q3A (impurities in drug substances) and Q3B (impurities in drug products) set clear limits on acceptable impurity levels. Compliance with these standards is mandatory to bring a new hormonal drug to market.
Why Regulatory Compliance Is Key?
- Patient Safety: Impurities can pose toxic risks to patients. For example, genotoxic impurities can cause DNA damage even at low concentrations.
- Drug Stability: Impurity profiling ensures that the drug remains safe throughout its intended shelf life.
- Manufacturing Quality: A detailed understanding of impurity profiles helps pharmaceutical manufacturers optimize their production methods.
By adhering to these guidelines, pharmaceutical companies can mitigate risks, enhance product quality, and demonstrate commitment to delivering effective medicines for public health.
Methods for Identifying and Quantifying Etonogestrel Impurities
Advanced Analytical Techniques
Pharmaceutical scientists employ a range of sophisticated methods to identify and quantify impurities in etonogestrel drug substances and products. These include:
- High-Performance Liquid Chromatography (HPLC): A widely used method for impurity detection and quantification.
- Gas Chromatography (GC): Ideal for identifying volatile impurities.
- Mass Spectrometry (MS): Enables structural identification of unknown impurities.
- Fourier Transform Infrared Spectroscopy (FTIR): Useful for identifying functional groups in complex impurity profiles.
- NMR Spectroscopy: Enables a detailed understanding of molecular-level aspects of impurities.
Each method has its strengths, and choosing the right one depends on the impurity’s chemical properties and the stage of drug development.
The Impact of Etonogestrel Impurities on Drug Development
1. Impact on Drug Safety
Impurities can affect drug safety, even at low levels. For example:
- Degradation Impurities: May form during storage or distribution and could potentially harm patients.
- Residual Solvents: Toxic solvents used in synthesis must be carefully removed to avoid adverse effects.
2. Impact on Drug Efficacy
Impurity interactions with the active molecule may alter its pharmacological efficacy. This makes impurity management a critical factor in ensuring consistent therapeutic performance of etonogestrel-based drugs.
3. Impact on Market Credibility
The pharmaceutical industry is heavily regulated, and even minor quality issues can lead to recalls, regulatory bans, and a loss of consumer trust. Proactive impurity profiling builds a company’s reputation as a reliable provider of effective and safe hormonal therapies.
Aquigen Bio Sciences: Redefining Impurity Profiling in India
Etonogestrel impurity profiling is an indispensable part of modern hormonal drug development, ensuring patient safety, regulatory compliance, and consistent product quality. It requires a detailed understanding of the sources, types, and implications of impurities, as well as a proactive approach to managing them through advanced analytical techniques and high-quality reference standards.
At Aquigen Bio Sciences, we understand the intricacies and challenges associated with hormonal drug development. As the leading resource for etonogestrel impurity standards in India, we take a comprehensive approach to support our clients in achieving regulatory excellence and product innovation. Our team of experts collaborates closely with drug developers, offering reliable solutions that pave the way for safe, effective, and compliant etonogestrel-based medicines. Trust Aquigen Bio Sciences to take your drug development process to the next level. Get in touch today to share your Etonogestrel impurity standards requirements with us. We are here to help you make safe, compliant pharmaceutical products.