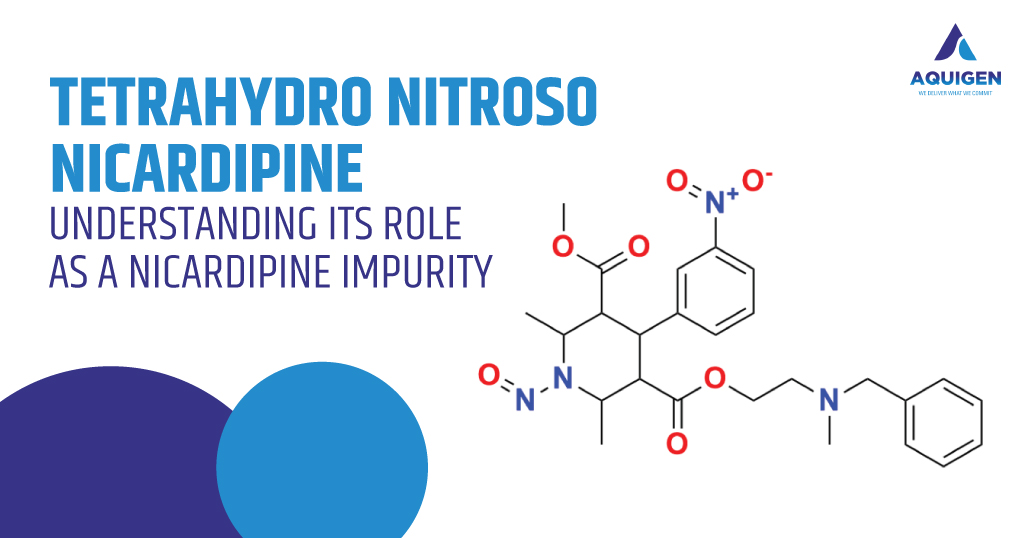
Nicardipine is a well-established calcium channel blocker utilized in the management of hypertension and angina. As with many therapeutic agents, the journey from synthesis to final pharmaceutical product involves intricate chemical transformations. During manufacturing, storage, or handling, a parent drug like Nicardipine can generate related substances known as impurities. Among these, Tetrahydro Nitroso Nicardipine has gained increasing attention due to its relevance in impurity profiling and pharmaceutical safety compliance.
At Aquigen Bio Sciences, the leading provider of impurity standards in India, we believe understanding impurities is critical for regulatory submissions, patient safety, and consistent drug performance. Identifying, isolating, and quantifying these related substances ensures the pharmaceutical product adheres to the highest safety and quality standards demanded by regulatory agencies worldwide. Let’s dive deeper into understanding Tetrahydro Nitroso Nicardipine in detail.
What is Tetrahydro Nitroso Nicardipine?
Tetrahydro Nitroso Nicardipine is classified as a process and degradation impurity of Nicardipine. It results from specific chemical reactions that occur during the synthesis or storage of Nicardipine. In pharmaceutical chemistry, nitroso derivatives are closely monitored due to their potential toxicological implications. The presence, identification, and control of nitroso impurities are a growing area of concern for global regulators and pharmaceutical manufacturers alike.
This impurity may form under oxidative or nitrosative conditions that Nicardipine could encounter during manufacturing or when exposed to certain stressors. The meticulous detection and quantification of Tetrahydro Nitroso Nicardipine are therefore essential steps in the pharmaceutical quality control workflow.
The Significance of Impurity Profiling in Pharmaceuticals
Impurities in drug substances are inevitable. Their sources include:
- The core synthetic route of the active pharmaceutical ingredient (API)
- Degradation byproducts from storage conditions
- Side reactions during manufacturing or purification processes
Impurity profiling involves characterizing these unwanted yet unavoidable molecules. Tetrahydro Nitroso Nicardipine, in this context, becomes a benchmark impurity for Nicardipine, helping scientists and regulators set permissible limits, refine production processes, and update analytical methods.
The European Medicines Agency (EMA), US Food and Drug Administration (FDA), International Council for Harmonisation (ICH), and other agencies have laid down stringent guidelines for the identification and quantification of pharmaceutical impurities. With nitroso impurities like Tetrahydro Nitroso Nicardipine, the urgency is even higher given their potential mutagenicity and regulatory scrutiny. Failure to control such impurities can lead to regulatory rejections, product recalls, and significant reputational damage. For manufacturers, this means that accessing reliable impurity standards is not just a matter of compliance but is also crucial in reducing toxicological risk and safeguarding patient health.
Analytical Methods for Detecting Nitroso Impurities
Analyzing and quantifying Tetrahydro Nitroso Nicardipine requires sophisticated analytical techniques. Techniques such as HPCL, LC-MS, and Gas Chromatography (GC) have become industry standards. Each of these methods offers high sensitivity and specificity, enabling the detection of trace levels of nitroso impurities.
Standardization of analytical reference materials is crucial for robust impurity identification. Pharmaceutical companies rely on highly pure, well-characterized impurity standards to calibrate instruments and validate their results. This ensures consistency and accuracy in impurity profiling, directly impacting drug safety.
Challenges in Sourcing and Characterizing Nitroso Nicardipine Standards
One of the key hurdles in impurity profiling is the acquisition of high-quality reference standards. Tetrahydro Nitroso Nicardipine Impurity, being a nitroso derivative, is often present at extremely low levels and may be unstable or difficult to synthesize.
This is where specialized providers like Aquigen Bio Sciences step in. As a contract research and manufacturing organization, Aquigen Bio Sciences bridges the gap by synthesizing and supplying high-purity pharmaceutical impurities, including process, degradation, and nitroso impurities, for research and regulatory purposes.
Why Partner with Aquigen Bio Sciences?
- Quality Assurance: All our impurity standards, including Tetrahydro Nitroso Nicardipine, are supplied with comprehensive characterization and supporting analytical data.
- Cutting-edge Infrastructure: Our laboratories utilize the latest in analytical and synthetic technologies ensuring reliability in product development.
- Regulatory Compliance: We prioritize thorough documentation and validation of each standard, aligning with global regulatory requirements.
- Expertise and Customization: Our team of skilled chemists addresses unique client requirements—custom synthesis, method development, and technical support are all core offerings.
- Research Focused: All products are designated for R&D or industrial use, aligning with our commitment to supporting innovation in drug discovery and development.
Choose The Right Partner for Nitroso Nicardipine Standards
In summary, Tetrahydro Nitroso Nicardipine plays a pivotal role in the impurity profiling of Nicardipine, guiding pharmaceutical companies toward higher safety and compliance. Effective detection and regulation of such impurities are vital for the pharmaceutical supply chain and ultimately, patient well-being.
When it comes to sourcing high-quality, fully characterized Nitroso Nicardipine impurity standards in India, Aquigen Bio Sciences sets the industry benchmark. We pride ourselves on being the leading provider, driven by our commitment to quality, innovation, and client satisfaction. Whether for regulatory submissions, R&D, or quality control, Aquigen delivers excellence and reliability for all your impurity standard requirements.
Explore our offerings and discover how we can empower your pharmaceutical research and compliance strategies. Choose Aquigen Bio Sciences, where quality and expertise shape the future of pharmaceutical research.
Explore more Nicardipine impurities
1.Tetrahydro Nitroso Nicardipine