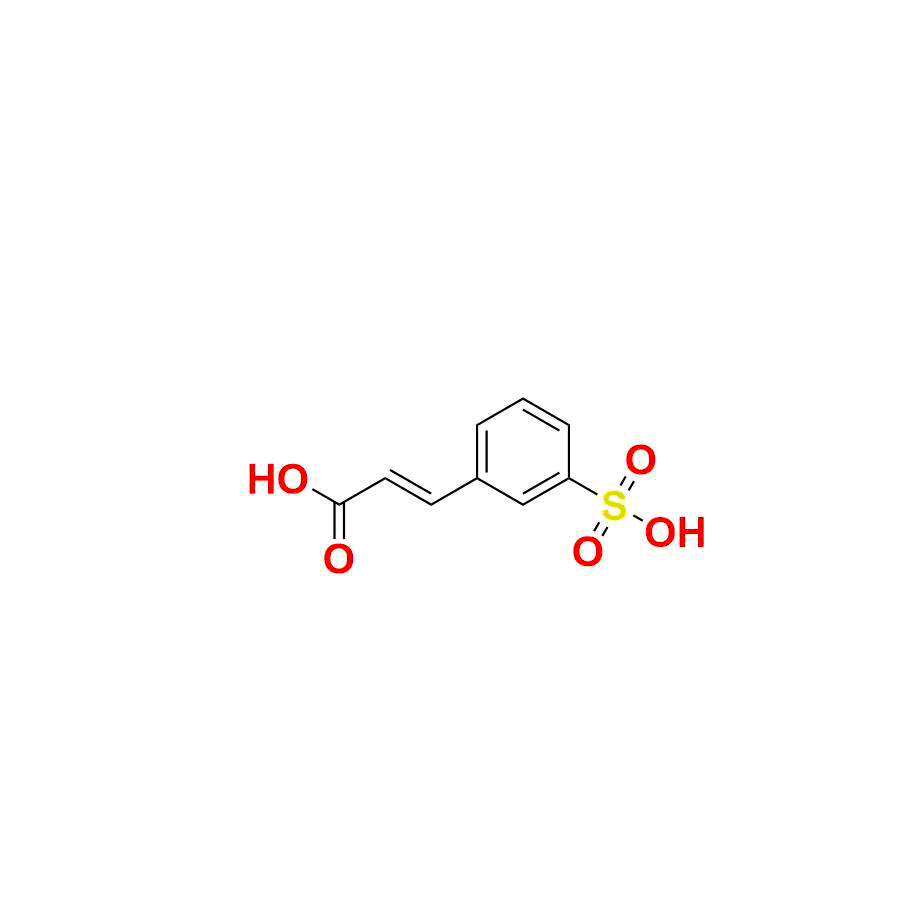
Belinostat Impurity 1
Available on backorder
| CAT NO | AQ-B001612 |
|---|---|
| CAS NO | 213125-46-3 |
| Mol.F | C9H8O5S |
| Mol.Wt | 228.2 |
| Availability | Custom Synthesis |
| Chemical name | (E)-3-(3-sulfophenyl)acrylic acid |
Ready To Dispatch Within 24 Hours
Available on backorder
Request for Quotation :
Belinostat Impurity 1
Product Description
Belinostat Impurity 1 is a high-quality reference standard supplied with comprehensive characterization data, ensuring compliance with regulatory guidelines. This product is essential for analytical method development, method validation (AMV), quality control (QC) applications, and plays a crucial role in Abbreviated New Drug Applications (ANDA).
Belinostat Impurity 1 can be used as a reference standard, with possible traceability to pharmacopeial standards such as USP or EP, based on feasibility. AquigenBio products are strictly for analytical purposes and is not intended for human use.
Applications of Belinostat Impurity 1:
1. Pharmaceutical Impurity Profiling
-
Belinostat Impurity 1 is a known process or degradation impurity of the oncology drug Belinostat.
-
Used to identify, quantify, and characterize impurity levels in Belinostat API and finished dosage forms.
2. Analytical Method Development & Validation
-
Essential for creating stability-indicating analytical methods such as:
-
HPLC
-
UPLC
-
LC-MS/MS
-
-
Assists in validating:
-
Specificity (distinguishing impurity from the main compound)
-
LOD/LOQ (Limit of Detection/Quantification)
-
Linearity, accuracy, and precision
-
3. Regulatory Compliance (ICH Q3A/B)
-
Used for regulatory submissions such as:
-
ANDA, DMF, and CTD dossiers
-
Supports ICH Q3A/B requirements for impurity limits and qualification
-
-
Provides toxicological data support when required for threshold exceedance.
4. Stability & Degradation Studies
-
Involved in forced degradation testing to track impurity formation under stress conditions (light, heat, humidity, pH).
-
Helps establish the impurity profile and contributes to shelf-life determination.
5. Quality Control (QC) & Batch Testing
-
Used in routine quality control labs for:
-
In-process monitoring
-
Batch release testing of Belinostat
-
Ensuring regulatory compliance and batch consistency
-
6. R&D and Impurity Pathway Studies
-
Supports synthetic route optimization and mechanistic impurity formation studies.
-
Applied in CROs, pharmaceutical R&D labs, and academic research for advanced impurity mapping.
⚠️ Note: Belinostat Impurity 1 is strictly for analytical, laboratory, and research use only. It is not intended for therapeutic or diagnostic use in humans or animals.
Safety & Handling Information for Chemical Products
1. Read the Safety Data Sheet (SDS) : The SDS contains important safety information for handling procedures, first-aid measures, disposal instructions, etc. Read it carefully.
2. Wear Personal Protective Equipment (PPE) : Always wear PPE as indicated in the SDS and by the manufacturer. This may include safety goggles, gloves, lab coats, respirators, and protective clothing.
3. Handle with Care : Be careful with chemical products - prevent spills, splashes, or inhaling fumes or dust. Use secondary trays or spill kits during chemical transfer or dispensing.
4. Work in a Well-Ventilated Area : Use chemical products in well-ventilated spaces to reduce vapour or gas exposure. Indoors, ensure ample ventilation via mechanical systems, fume hoods, or open windows and doors.
5. Avoid Mixing Chemicals : Avoid mixing chemical products unless directed by the manufacturer or a qualified professional. Incompatible mixes can lead to hazardous reactions, toxic gas releases, or explosions.
6. Labeling and Storage : Label chemical containers with product names, hazards, and handling information. Store in original or approved containers.
7. Prevent Contamination : Prevent cross-contamination with dedicated equipment or thorough decontamination. Keep food, drinks, and personal items away from chemical storage or work zones.
8. Emergency Procedures : Learn emergency procedures for spills, leaks, fires, or exposure incidents. Keep spill control materials, fire extinguishers, and emergency eyewash/shower facilities accessible.
9. First Aid : Know first aid for chemical exposure (eye contact, skin contact, inhalation, and ingestion). Ensure accessible supplies and trained personnel for assistance.
10. Proper Waste Disposal : Ensure chemical waste disposal as per local regulations; segregate hazardous from non-hazardous waste for health and environmental protection.
11. Training and Education : Offer thorough training to chemical handlers, covering usage, hazards, safety, and emergency response.
12. Report Incidents and Near Misses : Report incidents, near misses, spills, or safety concerns promptly to authorities and supervisors for investigation and action. By following these safety and handling guidelines, you can minimize risks associated with chemical products and create a safer work environment for yourself and others.
Read more
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.