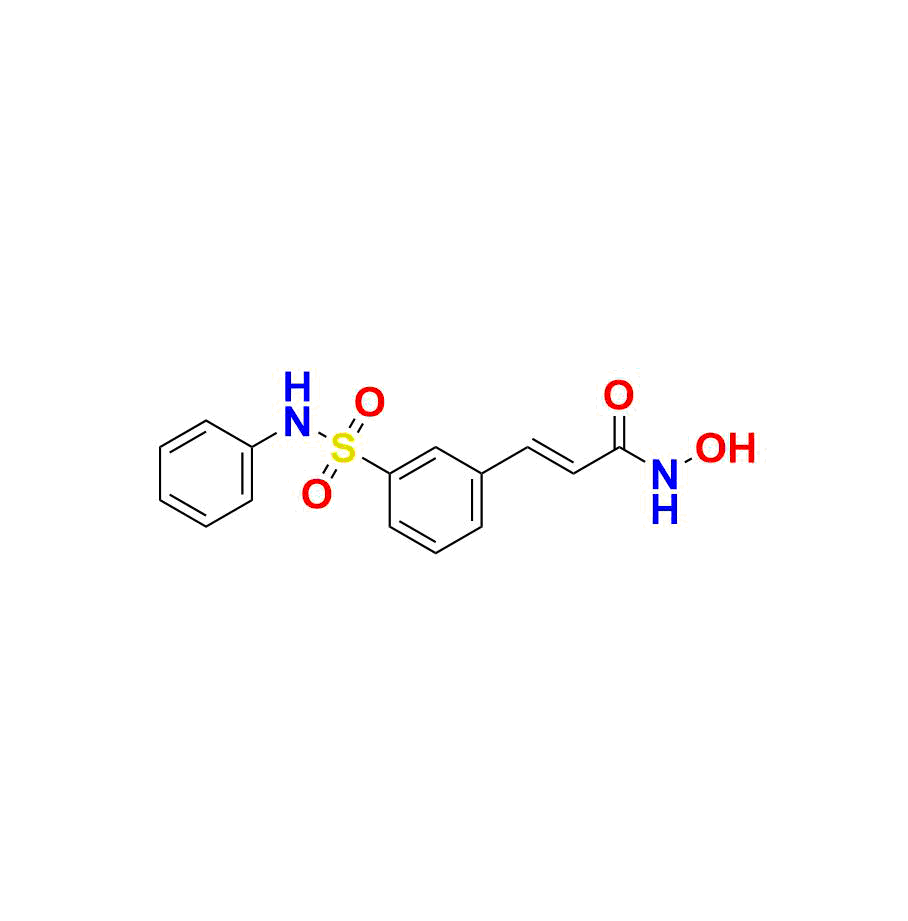
Belinostat
Available on backorder
| CAT NO | AQ-B001609 |
|---|---|
| CAS NO | 866323-14-0 |
| Mol.F | C15H14N2O4S |
| Mol.Wt | 318.3 |
| Availability | Custom Synthesis |
| Chemical name | (E)-N-hydroxy-3-(3-(N-phenylsulfamoyl)phenyl)acrylamide |
Ready To Dispatch Within 24 Hours
Available on backorder
Request for Quotation :
Belinostat
Product Description
Belinostat is a high-quality reference standard supplied with comprehensive characterization data, ensuring compliance with regulatory guidelines. This product is essential for analytical method development, method validation (AMV), quality control (QC) applications, and plays a crucial role in Abbreviated New Drug Applications (ANDA).
Belinostat can be used as a reference standard, with possible traceability to pharmacopeial standards such as USP or EP, based on feasibility. AquigenBio products are strictly for analytical purposes and is not intended for human use.
Applications of Belinostat:
1. Oncology Research and Drug Development
-
Belinostat is an FDA-approved histone deacetylase (HDAC) inhibitor used in the treatment of certain types of cancers, particularly:
-
Peripheral T-cell lymphoma (PTCL)
-
Other hematologic malignancies and solid tumors (in clinical studies)
-
-
Used to study epigenetic modulation in cancer cells, helping researchers explore gene expression regulation and tumor suppression mechanisms.
2. Pharmaceutical API Reference Standard
-
Employed as a reference standard in the development, validation, and quality control of Belinostat-based pharmaceutical formulations.
-
Utilized for:
-
Assay standardization
-
Impurity profiling
-
Stability testing
-
Method validation (HPLC, LC-MS/MS, etc.)
-
3. Epigenetic and Molecular Biology Research
-
A key tool in epigenetic drug screening and mechanistic studies involving:
-
Chromatin remodeling
-
Cell cycle arrest
-
Apoptosis induction in tumor cells
-
-
Facilitates research on the HDAC inhibition pathway, offering insights into cancer proliferation and differentiation.
4. Preclinical and Toxicological Studies
-
Used in in vitro and in vivo studies for:
-
Evaluating anti-cancer activity
-
Assessing pharmacokinetics and pharmacodynamics
-
Understanding dose-response and toxicity profiles
-
5. Formulation Development and Analytical Testing
-
Supports pharmaceutical R&D teams in designing oral or injectable dosage forms.
-
Helps in:
-
Developing bioanalytical methods for pharmacokinetic studies
-
Forced degradation and stability-indicating studies
-
Establishing analytical quality-by-design (AQbD) approaches
-
6. Clinical Research Applications
-
Belinostat is investigated in combination therapies with other chemotherapeutic agents, immunotherapies, or targeted therapies.
-
Supports studies aiming to:
-
Enhance therapeutic efficacy
-
Overcome drug resistance in cancers
-
Personalize cancer treatment based on epigenetic markers
-
⚠️ Note: Belinostat is a potent antineoplastic agent. It must be handled by trained professionals with appropriate safety precautions. Intended for research and pharmaceutical use only — not for human or veterinary consumption without regulatory approval.
Safety & Handling Information for Chemical Products
1. Read the Safety Data Sheet (SDS) : The SDS contains important safety information for handling procedures, first-aid measures, disposal instructions, etc. Read it carefully.
2. Wear Personal Protective Equipment (PPE) : Always wear PPE as indicated in the SDS and by the manufacturer. This may include safety goggles, gloves, lab coats, respirators, and protective clothing.
3. Handle with Care : Be careful with chemical products - prevent spills, splashes, or inhaling fumes or dust. Use secondary trays or spill kits during chemical transfer or dispensing.
4. Work in a Well-Ventilated Area : Use chemical products in well-ventilated spaces to reduce vapour or gas exposure. Indoors, ensure ample ventilation via mechanical systems, fume hoods, or open windows and doors.
5. Avoid Mixing Chemicals : Avoid mixing chemical products unless directed by the manufacturer or a qualified professional. Incompatible mixes can lead to hazardous reactions, toxic gas releases, or explosions.
6. Labeling and Storage : Label chemical containers with product names, hazards, and handling information. Store in original or approved containers.
7. Prevent Contamination : Prevent cross-contamination with dedicated equipment or thorough decontamination. Keep food, drinks, and personal items away from chemical storage or work zones.
8. Emergency Procedures : Learn emergency procedures for spills, leaks, fires, or exposure incidents. Keep spill control materials, fire extinguishers, and emergency eyewash/shower facilities accessible.
9. First Aid : Know first aid for chemical exposure (eye contact, skin contact, inhalation, and ingestion). Ensure accessible supplies and trained personnel for assistance.
10. Proper Waste Disposal : Ensure chemical waste disposal as per local regulations; segregate hazardous from non-hazardous waste for health and environmental protection.
11. Training and Education : Offer thorough training to chemical handlers, covering usage, hazards, safety, and emergency response.
12. Report Incidents and Near Misses : Report incidents, near misses, spills, or safety concerns promptly to authorities and supervisors for investigation and action. By following these safety and handling guidelines, you can minimize risks associated with chemical products and create a safer work environment for yourself and others.
Read more
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.