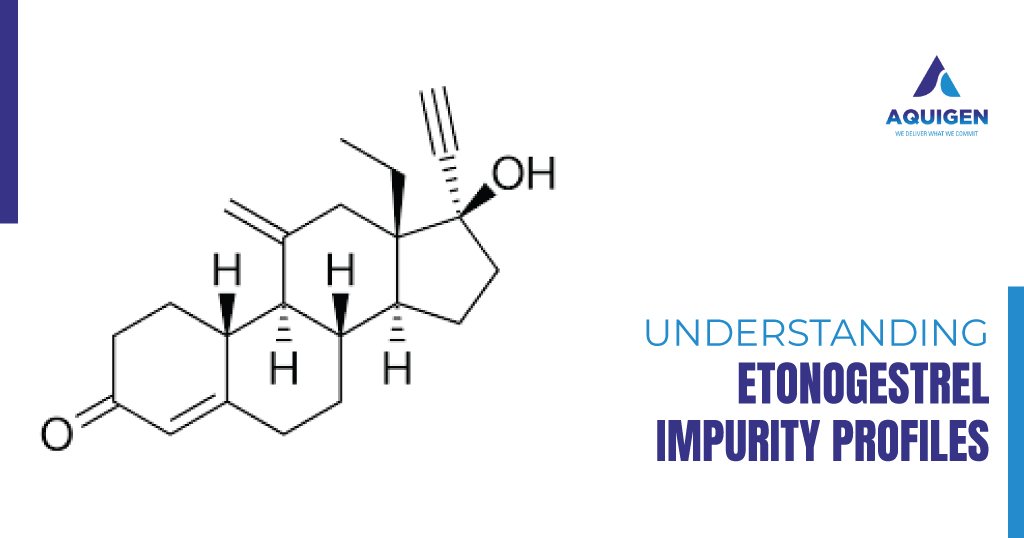
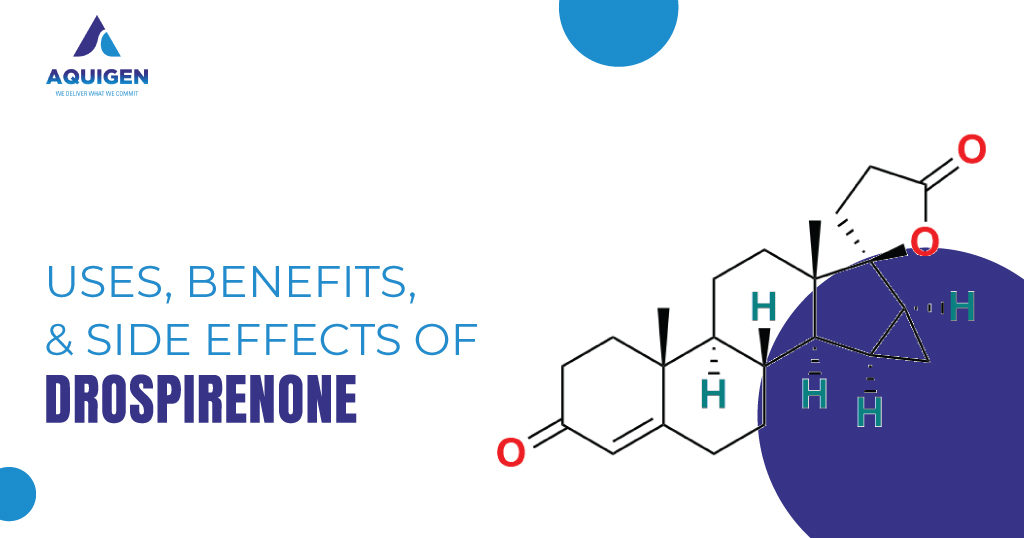
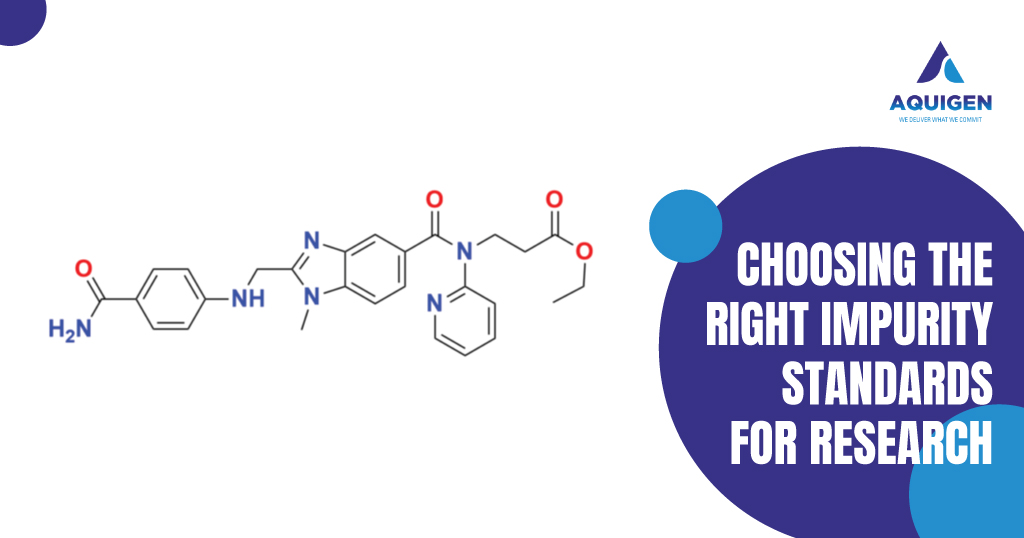
Key Challenges in Scaling Exemestane Manufacturing: How Impurity Standards Help
Exemestane, a groundbreaking therapeutic in hormone-dependent breast cancer treatment, has become a cornerstone drug within the pharmaceutical industry. Its complex manufacturing process, however, presents a multitude of challenges that directly impact production scalability, quality control, and compliance with regulatory guidelines. Manufacturers have to ensure the drug’s quality, safety, and purity while meeting tough global standards.
Key Challenges in Scaling Exemestane Manufacturing: How Impurity Standards Help Read More »