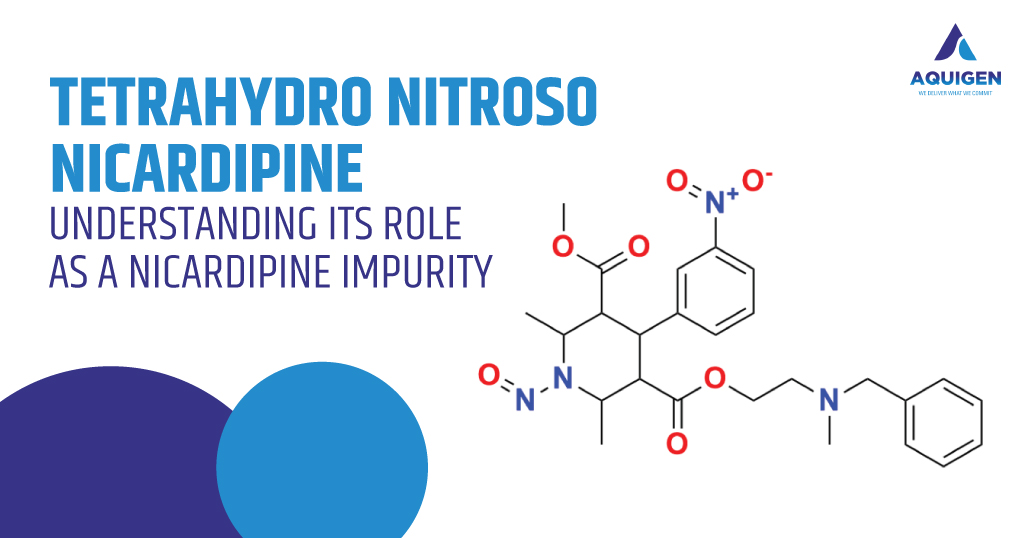
Tetrahydro Nitroso Nicardipine: Understanding Its Role as a Nicardipine Impurity
Nicardipine is a well-established calcium channel blocker utilized in the management of hypertension and angina. As with many therapeutic agents, the journey from synthesis to final pharmaceutical product involves intricate chemical transformations. During manufacturing, storage, or handling, a parent drug like Nicardipine can generate related substances known as impurities. Among these, Tetrahydro Nitroso Nicardipine has
Tetrahydro Nitroso Nicardipine: Understanding Its Role as a Nicardipine Impurity Read More »