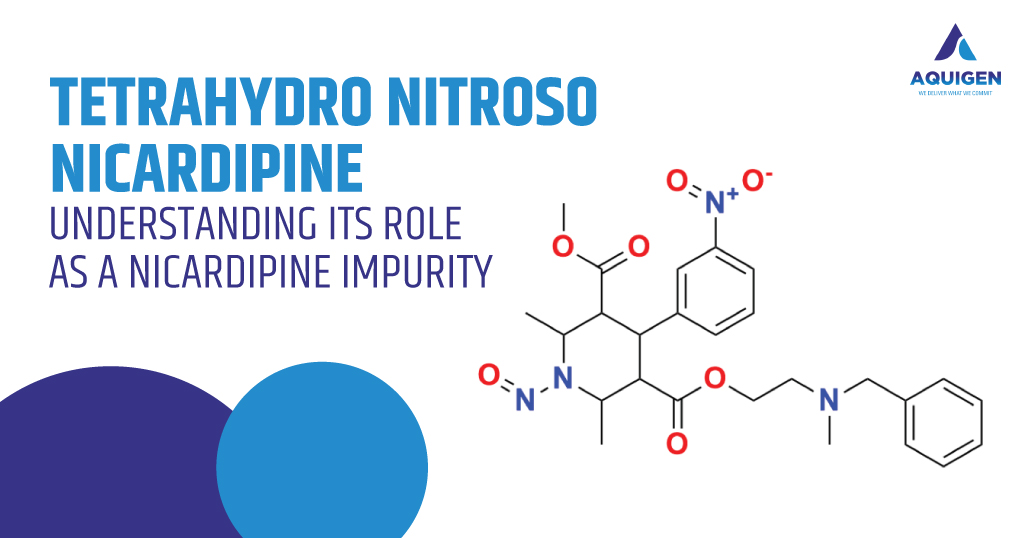
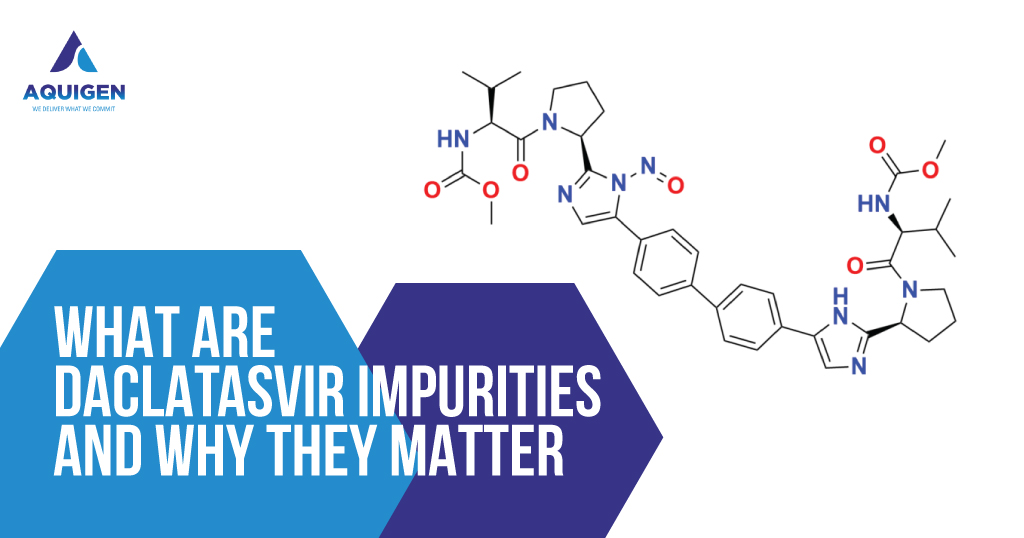
Introduction to Quetiapine and Its Pharmacological Role
Quetiapine is a widely used atypical antipsychotic, commonly prescribed for the management of various psychiatric disorders. Over the years, it has established a reputable position in psychopharmacology due to its unique mechanism of action, broad spectrum of therapeutic applications, and favourable safety profile relative to older antipsychotic agents. For contract research organizations and pharmaceutical manufacturers,
Introduction to Quetiapine and Its Pharmacological Role Read More »