Human Immunodeficiency Virus (HIV) has been a global health concern since its discovery in the early 1980s. The virus attacks the immune system, specifically the CD4 cells, making the body vulnerable to various opportunistic infections. Over the years, researchers have been working tirelessly to develop effective treatments to combat HIV and improve the quality of life for individuals living with the virus. One such breakthrough in HIV treatment is the development of Abacavir, a nucleoside reverse transcriptase inhibitor (NRTI) that has revolutionized the management of HIV infection. In this blog post, we will delve into the history, mechanism of action, efficacy, and side effects of Abacavir, as well as its impact on the lives of people living with HIV.
What is Abacavir?
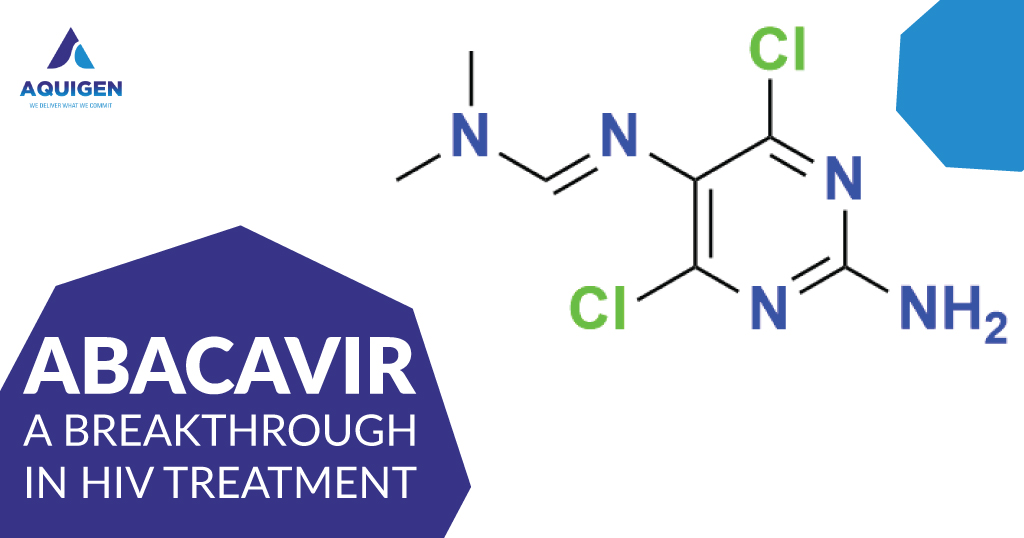
Abacavir is an antiretroviral medication used in combination with other drugs to treat HIV infection. It belongs to the class of drugs known as nucleoside reverse transcriptase inhibitors (NRTIs), which work by blocking the action of the enzyme reverse transcriptase, a crucial component in the replication process of HIV.
Abacavir was first discovered and developed by scientists at the Wellcome Research Laboratories, now part of GlaxoSmithKline, in the late 1990s. The drug was approved by the U.S. Food and Drug Administration (FDA) in 1998 and has since been used as a key component in various HIV treatment regimens.
Mechanism of Action
To understand how Abacavir works, it is essential to know the replication process of HIV. The virus enters the host cell and uses its enzyme, reverse transcriptase, to convert its RNA into DNA. This DNA then integrates into the host cell’s genome, allowing the virus to replicate and spread to other cells.
Abacavir, like other NRTIs, acts as a nucleoside analog, mimicking the building blocks of DNA. When the drug is incorporated into the growing DNA chain during the reverse transcription process, it leads to chain termination, preventing further elongation of the viral DNA. This disruption in the replication process effectively reduces the viral load in the body, allowing the immune system to recover and fight off opportunistic infections.
Need Abacavir Impurity Standards? Connect with Aquigen Bio Sciences- the leading Abacavir impurity standard manufacturer and supplier in Pune, India.
Efficacy and Clinical Studies
Numerous clinical studies have demonstrated the efficacy of Abacavir in the treatment of HIV infection. In a pivotal study called CNA3005, Abacavir was compared to placebo in combination with other antiretroviral drugs in treatment-naive patients. The results showed that the Abacavir-containing regimen significantly reduced viral load and increased CD4 cell counts compared to the placebo group.
Another landmark study, ACTG 5095, compared the efficacy of three different antiretroviral regimens, one of which contained Abacavir. The study found that the Abacavir-containing regimen was as effective as the other two regimens in suppressing viral load and increasing CD4 cell counts.
These studies, among others, have established Abacavir as a potent and effective antiretroviral drug in the management of HIV infection.
Side Effects and Precautions
Like all medications, Abacavir may cause side effects in some individuals. The most common side effects include:
- Nausea
- Headache
- Fatigue
- Diarrhea
- Skin rash
In rare cases, Abacavir can cause a severe hypersensitivity reaction, which can be life-threatening if not promptly recognized and treated. Symptoms of the hypersensitivity reaction may include fever, rash, fatigue, gastrointestinal symptoms, and respiratory symptoms. Individuals who develop a hypersensitivity reaction to Abacavir should discontinue the drug immediately and not restart it, as subsequent reactions can be more severe and potentially fatal.
To minimize the risk of hypersensitivity reactions, genetic testing is recommended before starting Abacavir therapy. Individuals who test positive for this allele are at a significantly higher risk of developing a hypersensitivity reaction and should not be prescribed Abacavir.
Impact on HIV Management
The introduction of Abacavir has had a significant impact on the management of HIV infection. As a potent antiretroviral drug with a favourable safety profile, Abacavir has become a cornerstone of many HIV treatment regimens.
One of the key advantages of Abacavir is its ability to penetrate the central nervous system (CNS), making it an important option for individuals with HIV-associated neurocognitive disorders. Additionally, Abacavir has been shown to have a lower risk of mitochondrial toxicity compared to some other NRTIs, which is a concern for long-term treatment.
The development of fixed-dose combination tablets containing Abacavir, such as Trizivir (Abacavir, Lamivudine, and Zidovudine) and Triumeq (Abacavir, Lamivudine, and Dolutegravir), has simplified HIV treatment regimens, improving adherence and quality of life for people living with HIV.
Conclusion
Abacavir has proven to be a breakthrough in the treatment of HIV infection, offering a potent and effective option for managing the virus. Its mechanism of action, targeting the reverse transcriptase enzyme, has been crucial in suppressing viral replication and allowing the immune system to recover. Clinical studies have consistently demonstrated the efficacy of Abacavir in reducing viral load and increasing CD4 cell counts, leading to improved health outcomes for people living with HIV.
While side effects, particularly the risk of hypersensitivity reactions, are a concern with Abacavir, genetic testing and careful monitoring have helped minimize these risks. The impact of Abacavir on HIV management has been significant, providing a valuable tool in the fight against the virus and improving the lives of countless individuals affected by HIV.
As the top Abacavir impurity standard manufacturer and supplier in Pune, India, Aquigen Bio Sciences is committed to supporting the pharmaceutical industry in the development and quality control of antiretroviral drugs like Abacavir. With our expertise in impurity synthesis and characterization, we provide high-quality reference standards that enable manufacturers to ensure the safety, efficacy, and purity of their products. By partnering with Aquigen Bio Sciences, pharmaceutical companies can rely on a trusted source for their impurity standard needs, ultimately contributing to the ongoing success of HIV treatment and the well-being of people living with the virus. Feel free to get in touch with us for your Abacavir impurity standard requirements.