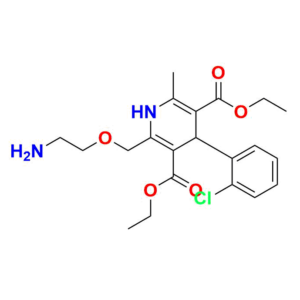
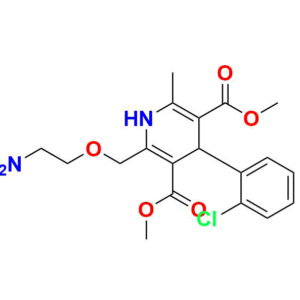
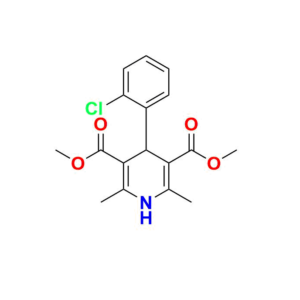
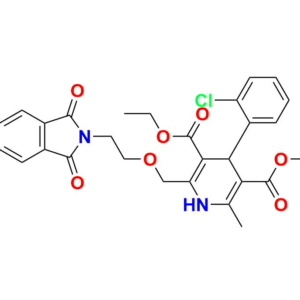
Pioneering Solutions for
Pharmaceutical Impurites
Your trusted source for Pharmaceutical Reference Standards
Precision at the Heart of Every Innovation
Accelerating Pharmaceutical innovation through our molecular expertise
Peptide Impurity Standards
Standards for the purity of peptide-based drugs during development.
At Aquigen BioSciences, we understand your search for a perfect product that fits your research requirements. We provides various services to support pharmaceutical research and development. We offer a wide range of Impurity Standards to help researchers test drug purity. Our services contain Deuterated Labelled Compounds which are useful for metabolism studies. Moreover, we supply Peptide Impurity Standards for peptide drug development. Aquigen undertakes custom synthesis projects to manufacture APIs, drug intermediates, and other molecules as required by customers. We have capabilities in API Intermediate and Custom Synthesis to optimize synthetic routes for new drug candidates. In addition, analytical services such as impurity isolation and characterization are available.
Other Services We Offer
- NitrosamineNitrosamine
- Medicinal ChemistryMedicinal Chemistry
- Analytical ServicesAnalytical Services
Precision at the Heart of Every Innovation
Accelerating Pharmaceutical innovation through our molecular expertise
Other Services We Offer
- NitrosamineNitrosamine
- Medicinal ChemistryMedicinal Chemistry
- Analytical ServicesAnalytical Services
Precision at the Heart of Every Innovation
Experience precision and trust in every test, backed by our accredited lab certifications




Leading the Way in Bio Science Advancements
Leading the Way in Bio Science Advancements
We are also a leading provider of Pharmaceutical Impurities like Degradation Impurities, Process Impurities, Building Blocks, Deuterated Isotopes Metabolites and Medicinal Chemistry molecules etc. Beyond commercially available products, we also accept custom orders for synthesis projects and offer analytical services like impurity isolation and characterization.
Aquigen Bio is your go-to partner for all the needs for breakthrough revolutions, improving human health and welfare in a lawful and conscientious manner.
- ISO/IEC - 17025:2017 Certified

- ISO - 9001:2015 Certified

- ISO - 17034:2016 Accredited

- MSME ( MICRO, SMALL & MEDIUM ENTERPRISES) Certified